 Found 16 hits for monomerid = 50187665
Found 16 hits for monomerid = 50187665 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50187665
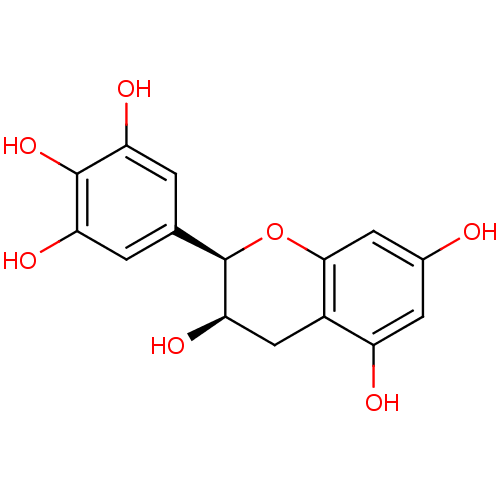
((-)-epigallocatechin | CHEMBL47386)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15-/m1/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| >1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of NLWAAQRYGRELRRMSD-K(FITC)-FVD from Bcl-2 (unknown origin) by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1007/s00044-009-9233-5
BindingDB Entry DOI: 10.7270/Q27M0BVB |
More data for this
Ligand-Target Pair | |
Enoyl-acyl-carrier protein reductase
(Plasmodium falciparum) | BDBM50187665

((-)-epigallocatechin | CHEMBL47386)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 17.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Immunology
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum ENR in presence of triclosan |
J Med Chem 50: 765-75 (2007)
Article DOI: 10.1021/jm061154d
BindingDB Entry DOI: 10.7270/Q2QJ7J4Q |
More data for this
Ligand-Target Pair | |
Enoyl-acyl-carrier protein reductase
(Plasmodium falciparum) | BDBM50187665

((-)-epigallocatechin | CHEMBL47386)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Immunology
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum ENR using crotonyl-CoA substrate |
J Med Chem 50: 765-75 (2007)
Article DOI: 10.1021/jm061154d
BindingDB Entry DOI: 10.7270/Q2QJ7J4Q |
More data for this
Ligand-Target Pair | |
Enoyl-acyl-carrier protein reductase
(Plasmodium falciparum) | BDBM50187665

((-)-epigallocatechin | CHEMBL47386)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Immunology
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum ENR using NADH substrate |
J Med Chem 50: 765-75 (2007)
Article DOI: 10.1021/jm061154d
BindingDB Entry DOI: 10.7270/Q2QJ7J4Q |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50187665

((-)-epigallocatechin | CHEMBL47386)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Concordia University Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from CB1 receptor (unknown origin) |
J Nat Prod 82: 636-646 (2019)
Article DOI: 10.1021/acs.jnatprod.8b00874 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50187665

((-)-epigallocatechin | CHEMBL47386)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.61E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Concordia University Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from CB2 receptor (unknown origin) |
J Nat Prod 82: 636-646 (2019)
Article DOI: 10.1021/acs.jnatprod.8b00874 |
More data for this
Ligand-Target Pair | |
6-phosphogluconate dehydrogenase (6PGD)
(Homo sapiens (Human)) | BDBM50187665

((-)-epigallocatechin | CHEMBL47386)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 6PGD |
Bioorg Med Chem 16: 3580-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.030
BindingDB Entry DOI: 10.7270/Q2F190K1 |
More data for this
Ligand-Target Pair | |
Glucose-6-phosphate 1-dehydrogenase
(Saccharomyces cerevisiae S288c) | BDBM50187665

((-)-epigallocatechin | CHEMBL47386)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation
Curated by ChEMBL
| Assay Description
Inhibition of yeast G6PD |
Bioorg Med Chem 16: 3580-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.030
BindingDB Entry DOI: 10.7270/Q2F190K1 |
More data for this
Ligand-Target Pair | |
Enoyl-ACP Reductase (FabI)
(Escherichia coli) | BDBM50187665

((-)-epigallocatechin | CHEMBL47386)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Immunology
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ENR |
J Med Chem 50: 765-75 (2007)
Article DOI: 10.1021/jm061154d
BindingDB Entry DOI: 10.7270/Q2QJ7J4Q |
More data for this
Ligand-Target Pair | |
Aldose reductase
(Rattus norvegicus) | BDBM50187665

((-)-epigallocatechin | CHEMBL47386)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of aldose reductase in rat lens homogenate |
J Nat Prod 66: 1191-6 (2003)
Article DOI: 10.1021/np0301543
BindingDB Entry DOI: 10.7270/Q2PV6M79 |
More data for this
Ligand-Target Pair | |
Enoyl-acyl-carrier protein reductase
(Plasmodium falciparum) | BDBM50187665

((-)-epigallocatechin | CHEMBL47386)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Immunology
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum ENR |
J Med Chem 50: 765-75 (2007)
Article DOI: 10.1021/jm061154d
BindingDB Entry DOI: 10.7270/Q2QJ7J4Q |
More data for this
Ligand-Target Pair | |
Ribonuclease H
(Escherichia coli (strain K12)) | BDBM50187665

((-)-epigallocatechin | CHEMBL47386)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli recombinant ribonuclease H after 30 mins by FRET quenching assay |
Bioorg Med Chem Lett 21: 2840-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.091
BindingDB Entry DOI: 10.7270/Q2H70G48 |
More data for this
Ligand-Target Pair | |
Anthrax toxin receptor 2
(Homo sapiens) | BDBM50187665

((-)-epigallocatechin | CHEMBL47386)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of CMG2 (40 to 217) C175A and R40C double mutant (unknown origin) interaction to full length PA E733C mutant expressed in Escherichia coli... |
J Med Chem 56: 1940-5 (2013)
Article DOI: 10.1021/jm301558t
BindingDB Entry DOI: 10.7270/Q2BP05Q3 |
More data for this
Ligand-Target Pair | |
Ribonuclease H
(Escherichia coli (strain K12)) | BDBM50187665

((-)-epigallocatechin | CHEMBL47386)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli recombinant ribonuclease H after 30 mins by FRET quenching assay |
Bioorg Med Chem Lett 21: 2840-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.091
BindingDB Entry DOI: 10.7270/Q2H70G48 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1/beta type-5
(Homo sapiens (Human)) | BDBM50187665

((-)-epigallocatechin | CHEMBL47386)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Agricultural and Food Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of purified human 20S proteasome assessed as decrease in AMC hydrolysis using Suc-Leu-Leu-Val-Tyr-AMC as sub... |
Eur J Med Chem 167: 291-311 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.044 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM50187665

((-)-epigallocatechin | CHEMBL47386)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of xanthine oxidase assessed as decrease in uric acid production by spectrophotometry |
J Nat Prod 61: 71-6 (1998)
Article DOI: 10.1021/np970237h
BindingDB Entry DOI: 10.7270/Q29C6Z93 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data