

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50190520 (RS)-1-butyl-3-isobutyl-9-(4-phenoxybenzyl)-1,4,9-triazaspiro[5.5]undecane-2,5-dione hydrochloride::1-butyl-3-(2-methylpropyl)-9-(4-phenoxyphenylmethyl)-1,4,9-triazaspiro[5.5]undeca-2,5-dione hydrochloride::1-butyl-3-isobutyl-9-(4-phenoxy-benzyl)-1,4,9-triaza-spiro[5.5]undecane-2,5-dione hydrochloride::CHEMBL537424::rac-1-Butyl-3-isobutyl-9-(4-phenoxybenzyl)-1,4,9-triazaspiro[5.5]undecane-2,5-dione hydrochloride
SMILES: CCCCN1C(=O)C(CC(C)C)NC(=O)C11CCN(Cc2ccc(Oc3ccccc3)cc2)CC1
InChI Key: InChIKey=SBPSJQLYVXFZFV-UHFFFAOYSA-N
Data: 15 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50190520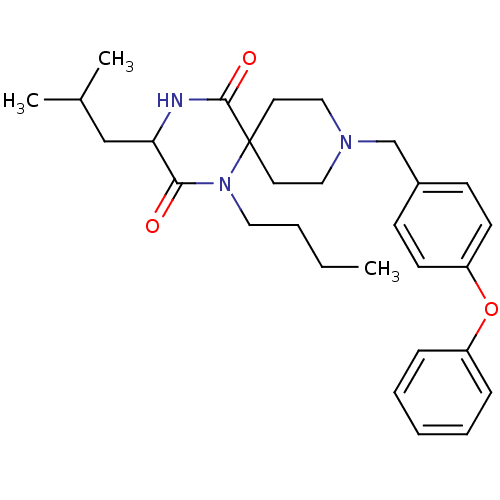 ((RS)-1-butyl-3-isobutyl-9-(4-phenoxybenzyl)-1,4,9-...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 851 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human CCR5 expressed in HOS cells assessed as inhibition of cell fusion with HIV gp120 expressing HEK293 cells by LTR lucifera... | Bioorg Med Chem Lett 21: 1141-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.109 BindingDB Entry DOI: 10.7270/Q24T6JP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50190520 ((RS)-1-butyl-3-isobutyl-9-(4-phenoxybenzyl)-1,4,9-...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CCR5 receptor expressed in CHO cells assessed as inhibition of MIP-1-alpha-stmulated calcium mobilization | Bioorg Med Chem 18: 5208-23 (2010) Article DOI: 10.1016/j.bmc.2010.05.057 BindingDB Entry DOI: 10.7270/Q25H7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50190520 ((RS)-1-butyl-3-isobutyl-9-(4-phenoxybenzyl)-1,4,9-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of CYP2C9 | Bioorg Med Chem Lett 21: 1141-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.109 BindingDB Entry DOI: 10.7270/Q24T6JP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50190520 ((RS)-1-butyl-3-isobutyl-9-(4-phenoxybenzyl)-1,4,9-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 21: 1141-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.109 BindingDB Entry DOI: 10.7270/Q24T6JP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50190520 ((RS)-1-butyl-3-isobutyl-9-(4-phenoxybenzyl)-1,4,9-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | Bioorg Med Chem Lett 21: 1141-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.109 BindingDB Entry DOI: 10.7270/Q24T6JP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50190520 ((RS)-1-butyl-3-isobutyl-9-(4-phenoxybenzyl)-1,4,9-...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human CCR5 expressed in CHO cells assessed as inhibition of MIP-1alpha-induced calcium mobilization Ca assay | Bioorg Med Chem Lett 21: 1141-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.109 BindingDB Entry DOI: 10.7270/Q24T6JP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50190520 ((RS)-1-butyl-3-isobutyl-9-(4-phenoxybenzyl)-1,4,9-...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human CCR5 expressed in CHO cells assessed as inhibition of MIP-1-alpha-stimulated calcium mobilization | Bioorg Med Chem Lett 17: 727-31 (2007) Article DOI: 10.1016/j.bmcl.2006.10.084 BindingDB Entry DOI: 10.7270/Q2XK8GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50190520 ((RS)-1-butyl-3-isobutyl-9-(4-phenoxybenzyl)-1,4,9-...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [125I]MIP1-alpha from human CCR5 expressed in CHO cells | Bioorg Med Chem Lett 17: 727-31 (2007) Article DOI: 10.1016/j.bmcl.2006.10.084 BindingDB Entry DOI: 10.7270/Q2XK8GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (CXCR4) (Homo sapiens (Human)) | BDBM50190520 ((RS)-1-butyl-3-isobutyl-9-(4-phenoxybenzyl)-1,4,9-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of radio-isotope labeled SDF1-alpha binding to human recombinant CXCR4 | J Med Chem 49: 4140-52 (2006) Article DOI: 10.1021/jm060051s BindingDB Entry DOI: 10.7270/Q2ZG6T1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50190520 ((RS)-1-butyl-3-isobutyl-9-(4-phenoxybenzyl)-1,4,9-...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of radio-isotope labeled MIP1-alpha binding to human recombinant CCR5 | J Med Chem 49: 4140-52 (2006) Article DOI: 10.1021/jm060051s BindingDB Entry DOI: 10.7270/Q2ZG6T1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50190520 ((RS)-1-butyl-3-isobutyl-9-(4-phenoxybenzyl)-1,4,9-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of radio-isotope labeled MCP1 binding to human recombinant CCR2 | J Med Chem 49: 4140-52 (2006) Article DOI: 10.1021/jm060051s BindingDB Entry DOI: 10.7270/Q2ZG6T1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50190520 ((RS)-1-butyl-3-isobutyl-9-(4-phenoxybenzyl)-1,4,9-...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity against human recombinant CCR5 expressed in CHO cells assessed as inhibition of human MIP-1-alpha-stimulated calcium mobilization | J Med Chem 49: 4140-52 (2006) Article DOI: 10.1021/jm060051s BindingDB Entry DOI: 10.7270/Q2ZG6T1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50190520 ((RS)-1-butyl-3-isobutyl-9-(4-phenoxybenzyl)-1,4,9-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity against human recombinant CCR2 expressed in CHO cells assessed as inhibition of human MCP1-stimulated calcium mobilization | J Med Chem 49: 4140-52 (2006) Article DOI: 10.1021/jm060051s BindingDB Entry DOI: 10.7270/Q2ZG6T1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (CXCR4) (Homo sapiens (Human)) | BDBM50190520 ((RS)-1-butyl-3-isobutyl-9-(4-phenoxybenzyl)-1,4,9-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity against human recombinant CXCR4 expressed in CHO cells assessed as inhibition of human SDF1-alpha-stimulated calcium mobilization | J Med Chem 49: 4140-52 (2006) Article DOI: 10.1021/jm060051s BindingDB Entry DOI: 10.7270/Q2ZG6T1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50190520 ((RS)-1-butyl-3-isobutyl-9-(4-phenoxybenzyl)-1,4,9-...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CCR5 receptor overexpressed in CHO cells assessed as inhibition of MIP1alpha-induced calcium mobilization | Bioorg Med Chem Lett 20: 763-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.018 BindingDB Entry DOI: 10.7270/Q2NK3F4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||