

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50191347 CHEMBL3907376
SMILES: C[C@H]1COC(=O)Nc2cccc(CN(C)C(=O)[C@H](Nc3ccc4c(N)nccc4c3)c3ccc1c(C)c3)c2
InChI Key: InChIKey=WGWNPJIMCIPPBO-UZTOHYMASA-N
Data: 5 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor III/Factor VIIa (fVIIa) (Homo sapiens (Human)) | BDBM50191347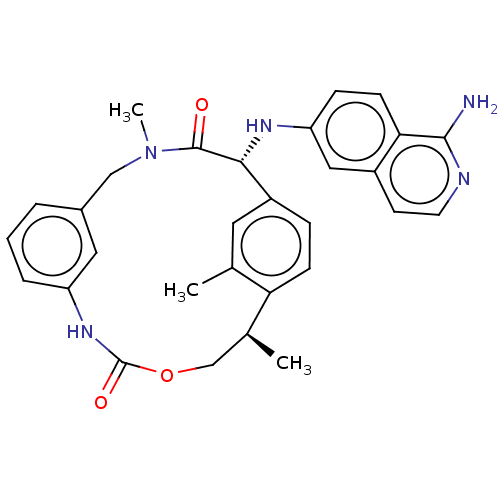 (CHEMBL3907376) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of full-length human TF/recombinant human factor 7a assessed as decrease in conversion of factor 10 to factor 10a by measuring S2765 hydro... | J Med Chem 59: 7125-37 (2016) BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM50191347 (CHEMBL3907376) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human HK1 using H-D-Val-Leu-Arg-AFC as substrate assessed as release of AFC after 10 to 120 mins by spectrofluorimetric method | J Med Chem 59: 7125-37 (2016) BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50191347 (CHEMBL3907376) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyroGlu-Pro-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectrophotometric method | J Med Chem 59: 7125-37 (2016) BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50191347 (CHEMBL3907376) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human factor 10a using N-benzoyl-Ile-Glu-(OH,OMe)-Gly-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectroph... | J Med Chem 59: 7125-37 (2016) BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50191347 (CHEMBL3907376) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of alpha-thrombin (unknown origin) using pyroGlu-Pro-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectrophotom... | J Med Chem 59: 7125-37 (2016) BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||