

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50192315 CHEMBL3981856::US10239870, Example 287
SMILES: Cn1c(SCCCN2CC[C@]3(C[C@@H]3c3ccc(cc3)C(F)(F)F)C2)nnc1-c1ccc(CC(N)=O)cc1
InChI Key: InChIKey=BCTUVZJFJIFLND-GJZUVCINSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50192315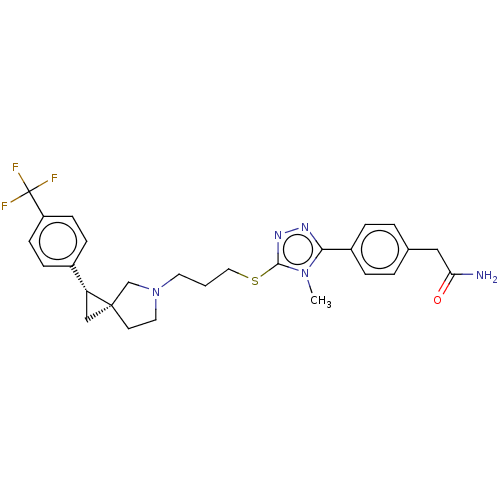 (CHEMBL3981856 | US10239870, Example 287) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description [125I]-7OH-PIPAT Binding Assay at rat native D3 receptor on membranes from rat ventral striatum. Homogenates from frozen rat brain ventral striatum (... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2HT2RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50192315 (CHEMBL3981856 | US10239870, Example 287) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D3 receptor expressed in CHO-K1 cell membranes after 90 mins by liquid scintillation counting | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine D2 S receptor (Homo sapiens (Human)) | BDBM50192315 (CHEMBL3981856 | US10239870, Example 287) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 468 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description CHO cells stably expressing human dopamine receptor type 2, long variant (hD2L), coupled to Gα16 protein (CHO-Gα16-hD2L) were re-suspended ... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2HT2RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50192315 (CHEMBL3981856 | US10239870, Example 287) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 468 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2 receptor expressed in CHO-K1 cell membranes coexpressing Galpha16 after 120 mins by liquid scin... | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM50192315 (CHEMBL3981856 | US10239870, Example 287) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 expressed in microsomes using ER as substrate after 10 mins by P450 cypex assay | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50192315 (CHEMBL3981856 | US10239870, Example 287) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 expressed in microsomes using FCA as substrate after 10 mins by P450 cypex assay | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50192315 (CHEMBL3981856 | US10239870, Example 287) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in microsomes using DEF as substrate after 10 mins by P450 cypex assay | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50192315 (CHEMBL3981856 | US10239870, Example 287) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Inhibition of human CYP2C19 expressed in microsomes using BMC as substrate after 10 mins by P450 cypex assay | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50192315 (CHEMBL3981856 | US10239870, Example 287) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Inhibition of human ERG transfected in HEK293 cells assessed as reduction in tail current by patch clamp assay | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50192315 (CHEMBL3981856 | US10239870, Example 287) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in microsomes using MMC as substrate after 10 mins by P450 cypex assay | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||