

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50192319 CHEMBL3920167::US10239870, Example 279
SMILES: Cn1c(SCCCN2CC[C@]3(C[C@@H]3c3ccc(cc3)C(F)(F)F)C2)nnc1-c1cccc(c1)-c1ncco1
InChI Key: InChIKey=NMEPMLDUQJFQOK-KCWPFWIISA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50192319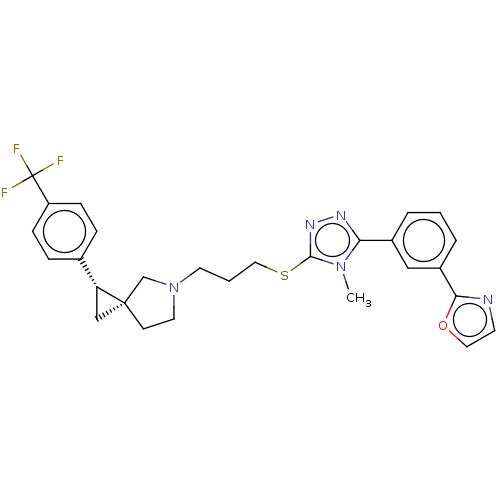 (CHEMBL3920167 | US10239870, Example 279) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D3 receptor expressed in CHO-K1 cell membranes after 90 mins by liquid scintillation counting | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50192319 (CHEMBL3920167 | US10239870, Example 279) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description [125I]-7OH-PIPAT Binding Assay at rat native D3 receptor on membranes from rat ventral striatum. Homogenates from frozen rat brain ventral striatum (... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2HT2RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50192319 (CHEMBL3920167 | US10239870, Example 279) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.363 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Antagonist activity at human dopamine D3 receptor expressed in CHO cell membranes after 90 mins in presence of quinelorane by [35S]-GTPgammaS binding... | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50192319 (CHEMBL3920167 | US10239870, Example 279) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2 receptor expressed in CHO-K1 cell membranes coexpressing Galpha16 after 120 mins by liquid scin... | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50192319 (CHEMBL3920167 | US10239870, Example 279) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description CHO cells stably expressing human dopamine receptor type 2, long variant (hD2L), coupled to Gα16 protein (CHO-Gα16-hD2L) were re-suspended ... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2HT2RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50192319 (CHEMBL3920167 | US10239870, Example 279) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Antagonist activity at human dopamine D2L receptor expressed in CHO cells coexpressing Galpha16 assessed as inhibition of dopamine-induced Ca2+ stimu... | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GSC2 (Saccharomyces cerevisiae) | BDBM50192319 (CHEMBL3920167 | US10239870, Example 279) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Inhibition of human ERG transfected in HEK293 cells assessed as reduction in tail current by patch clamp assay | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||