

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50194429 (-)-parthenolide::(1aR,7aS,10aS,10bS,Z)-1a,5-dimethyl-8-methylene-2,3,6,7,7a,8-hexahydro-11-oxa-bicyclo[8.1.0]undeca-1(10),4-dieno[9,8-b]furan-9(1aH,10aH,10bH)-one::(Z)-(1S,2S,4R,11S)-4,8-Dimethyl-12-methylene-3,14-dioxa-tricyclo[9.3.0.0*2,4*]tetradec-7-en-13-one::CHEMBL540445::PARTHENOLIDE
SMILES: C\C1=C\CC[C@@]2(C)O[C@H]2[C@H]2OC(=O)C(=C)[C@@H]2CC1
InChI Key: InChIKey=KTEXNACQROZXEV-QLIGOWBFSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HTR1A (RABBIT) | BDBM50194429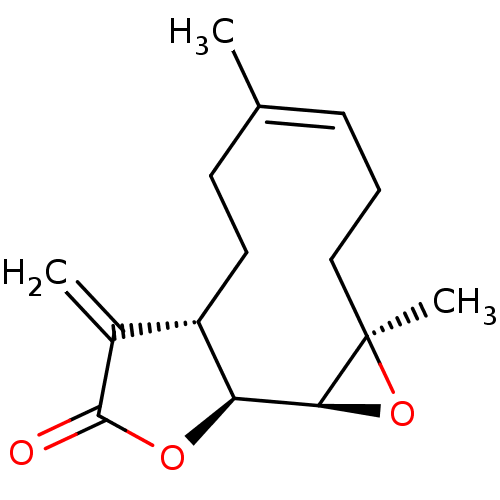 ((-)-parthenolide | (1aR,7aS,10aS,10bS,Z)-1a,5-dime...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana Curated by PDSP Ki Database | Comp Biochem Physiol C, Pharmacol Toxicol Endocrinol 117: 19-24 (1997) Article DOI: 10.1016/s0742-8413(97)00614-2 BindingDB Entry DOI: 10.7270/Q2PV6HWK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50194429 ((-)-parthenolide | (1aR,7aS,10aS,10bS,Z)-1a,5-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from rat 5HT2A receptor expressed in mouse NIH3T3 cells | J Nat Prod 60: 651-3 (1997) Article DOI: 10.1021/np960644d BindingDB Entry DOI: 10.7270/Q2ZC83R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50194429 ((-)-parthenolide | (1aR,7aS,10aS,10bS,Z)-1a,5-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A receptor in rat brain membrane | J Nat Prod 60: 651-3 (1997) Article DOI: 10.1021/np960644d BindingDB Entry DOI: 10.7270/Q2ZC83R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM50194429 ((-)-parthenolide | (1aR,7aS,10aS,10bS,Z)-1a,5-dime...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of COX2 | J Nat Prod 68: 985-91 (2005) Article DOI: 10.1021/np049655u BindingDB Entry DOI: 10.7270/Q27S7PN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MurA (P. aeruginosa) (Pseudomonas aeruginosa (G-proteobacteria)) | BDBM50194429 ((-)-parthenolide | (1aR,7aS,10aS,10bS,Z)-1a,5-dime...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa PAO1293 MurA in presence of UNAG | Bioorg Med Chem Lett 16: 5605-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.021 BindingDB Entry DOI: 10.7270/Q20Z742G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MurA (E. coli) (Escherichia coli K-12 (Enterobacteria)) | BDBM50194429 ((-)-parthenolide | (1aR,7aS,10aS,10bS,Z)-1a,5-dime...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 Mur A in presence of UNAG | Bioorg Med Chem Lett 16: 5605-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.021 BindingDB Entry DOI: 10.7270/Q20Z742G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric Oxide Synthase, inducible (Mus musculus (mouse)) | BDBM50194429 ((-)-parthenolide | (1aR,7aS,10aS,10bS,Z)-1a,5-dime...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Inhibition of iNOS in LPS-induced mouse RAW264.7 cells assessed as inhibition of nitric acid at measured after 24 hrs | J Nat Prod 76: 679-84 (2013) Article DOI: 10.1021/np300893n BindingDB Entry DOI: 10.7270/Q2ZW1N83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||