

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50199178 1-beta-D-ribofuranosyl(3H)pyrimidine-2,4,6-trione 5'-monophosphate::3,4-dihydroxy-5-(6-oxido-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-2-yl)methyl phosphate::6-HYDROXYURIDINE-5'-PHOSPHATE::6-hydroxy-UMP::CHEMBL383923
SMILES: O[C@@H]1[C@@H](COP(O)(O)=O)O[C@H]([C@@H]1O)n1c(O)cc(=O)[nH]c1=O
InChI Key: InChIKey=UDOBICLZEKUKCV-YXZULKJRSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orotidine phosphate decarboxylase (Saccharomyces cerevisiae) | BDBM50199178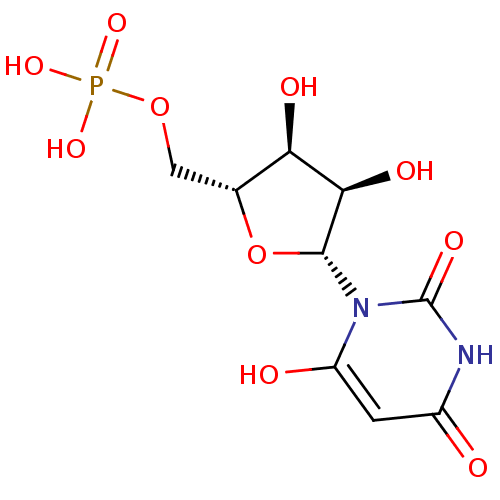 (1-beta-D-ribofuranosyl(3H)pyrimidine-2,4,6-trione ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisia uridine 5'-monophosphate synthase | Bioorg Med Chem 18: 4032-41 (2010) Article DOI: 10.1016/j.bmc.2010.04.017 BindingDB Entry DOI: 10.7270/Q24T6KBX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine phosphate decarboxylase (Saccharomyces cerevisiae) | BDBM50199178 (1-beta-D-ribofuranosyl(3H)pyrimidine-2,4,6-trione ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00900 | -15.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University Health Network Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisia uridine 5'-monophosphate synthase after overnight incubation at room temperature by VP-ITC microcalorimetry | Bioorg Med Chem 18: 4032-41 (2010) Article DOI: 10.1016/j.bmc.2010.04.017 BindingDB Entry DOI: 10.7270/Q24T6KBX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pyrimidinergic receptor P2Y6 (Homo sapiens (Human)) | BDBM50199178 (1-beta-D-ribofuranosyl(3H)pyrimidine-2,4,6-trione ...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyrimidinergic receptor P2Y4 (Homo sapiens (Human)) | BDBM50199178 (1-beta-D-ribofuranosyl(3H)pyrimidine-2,4,6-trione ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purinergic receptor P2Y2 (Homo sapiens (Human)) | BDBM50199178 (1-beta-D-ribofuranosyl(3H)pyrimidine-2,4,6-trione ...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||