

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50199521 16-methyl-14-[2-(4-{2-[16-methyl-5-oxo-6,14-diazatetracyclo[7.5.3.01,10.02,7]heptadeca-2(7),3,16-trien-14-yl]ethyl}-1,4-diazepan-1-yl)ethyl]-6,14-diazatetracyclo[7.5.3.01,10.02,7]heptadeca-2(7),3,16-trien-5-one::CHEMBL389583::N,N'-bis(1-oxo-8,15-didehydrolycodinoethyl)-homopiperazine
SMILES: CC1=C[C@H]2Cc3[nH]c(=O)ccc3[C@@]3(C1)[C@@H]2CCCN3CCN1CCCN(CCN2CCC[C@@H]3[C@@H]4Cc5[nH]c(=O)ccc5[C@]23CC(C)=C4)CC1
InChI Key: InChIKey=HJNXIXULSCHZBG-YEUCHJDASA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50199521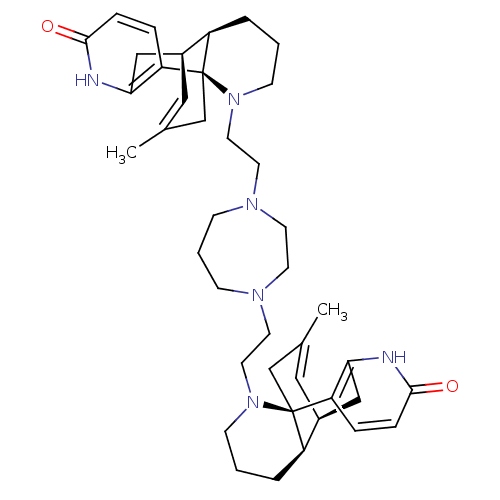 (16-methyl-14-[2-(4-{2-[16-methyl-5-oxo-6,14-diazat...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against butyrylcholinesterase from rat serum | Bioorg Med Chem Lett 15: 523-6 (2005) Article DOI: 10.1016/j.bmcl.2004.11.060 BindingDB Entry DOI: 10.7270/Q2Z89BW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50199521 (16-methyl-14-[2-(4-{2-[16-methyl-5-oxo-6,14-diazat...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 218 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex at pH 5 | Bioorg Med Chem 15: 1394-408 (2007) Article DOI: 10.1016/j.bmc.2006.11.009 BindingDB Entry DOI: 10.7270/Q27W6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50199521 (16-methyl-14-[2-(4-{2-[16-methyl-5-oxo-6,14-diazat...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of BuChE in rat serum | Bioorg Med Chem 15: 1394-408 (2007) Article DOI: 10.1016/j.bmc.2006.11.009 BindingDB Entry DOI: 10.7270/Q27W6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50199521 (16-methyl-14-[2-(4-{2-[16-methyl-5-oxo-6,14-diazat...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 218 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against acetylcholinesterase from rat cortex homogenate by ellman method was determined | Bioorg Med Chem Lett 15: 523-6 (2005) Article DOI: 10.1016/j.bmcl.2004.11.060 BindingDB Entry DOI: 10.7270/Q2Z89BW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||