

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50203985 (E)-1-(2,4-dihydroxyphenyl)-3-(2,4-dihydroxyphenyl)-2-propen-1-one (18)::2,2',4,4'-tetrahydroxychalcone::2,4,2',4'-tetrahydroxychalcone::CHEMBL394855
SMILES: Oc1ccc(\C=C\C(=O)c2ccc(O)cc2O)c(O)c1
InChI Key: InChIKey=ZWTDXYUDJYDHJR-QHHAFSJGSA-N
Data: 8 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50203985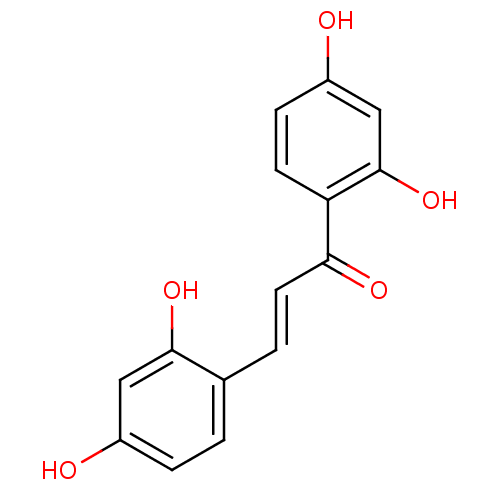 ((E)-1-(2,4-dihydroxyphenyl)-3-(2,4-dihydroxyphenyl...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | 4.5 | 37 |
Shanghai Institute of Materia Medica, Chinese Academy of Sciences | Assay Description The assay based on fluorescenceresonance energy transfer was carried out with BACE1 enzyme at pH 4.5 with a substrate, H-Lys(DABSYL)-SEVNLDAEFR-Gin-(... | J Enzyme Inhib Med Chem 26: 643-8 (2011) Article DOI: 10.3109/14756366.2010.543420 BindingDB Entry DOI: 10.7270/Q2DR2TCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Agaricus bisporus (Common mushroom)) | BDBM50203985 ((E)-1-(2,4-dihydroxyphenyl)-3-(2,4-dihydroxyphenyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase | Bioorg Med Chem 15: 2396-402 (2007) Article DOI: 10.1016/j.bmc.2007.01.017 BindingDB Entry DOI: 10.7270/Q2R78DWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldose reductase (Rattus norvegicus) | BDBM50203985 ((E)-1-(2,4-dihydroxyphenyl)-3-(2,4-dihydroxyphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Rattus norvegicus (rat) lens aldose reductase | Citation and Details Article DOI: 10.1007/s00044-012-0367-5 BindingDB Entry DOI: 10.7270/Q2XP77VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50203985 ((E)-1-(2,4-dihydroxyphenyl)-3-(2,4-dihydroxyphenyl...) | UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse B16 cells assessed as reduction in melanin synthesis after 1 hr | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50203985 ((E)-1-(2,4-dihydroxyphenyl)-3-(2,4-dihydroxyphenyl...) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50203985 ((E)-1-(2,4-dihydroxyphenyl)-3-(2,4-dihydroxyphenyl...) | UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse B16 cells assessed as reduction in melanin synthesis after 1 hr | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50203985 ((E)-1-(2,4-dihydroxyphenyl)-3-(2,4-dihydroxyphenyl...) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Agaricus bisporus (Common mushroom)) | BDBM50203985 ((E)-1-(2,4-dihydroxyphenyl)-3-(2,4-dihydroxyphenyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase assessed as reduction in diphenolase activity using L-DOPA substrate pre-incubated for 20 mins at 30 degC | Bioorg Med Chem 21: 2156-62 (2013) Article DOI: 10.1016/j.bmc.2012.12.054 BindingDB Entry DOI: 10.7270/Q28P63FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||