

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50204072 CHEMBL3945199::US10543183, Compound 12::US10945978, Compound 12
SMILES: CC(C)(O)c1cn(c(n1)C(C)(C)c1c(Cl)cccc1Cl)-c1ccc(cc1F)-c1cc(F)c(CO)c(c1)S(C)(=O)=O
InChI Key: InChIKey=HNAJDMYOTDNOBK-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retinoic acid receptor RXR-alpha/oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50204072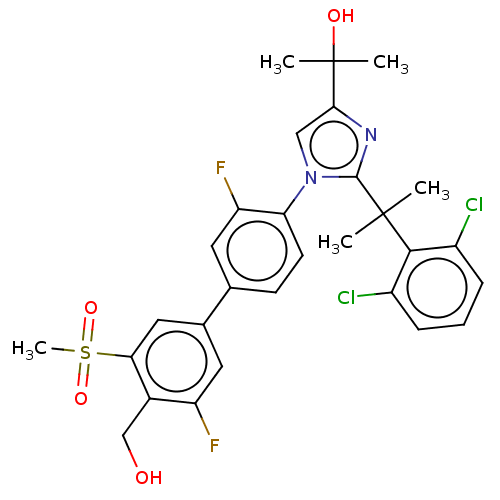 (CHEMBL3945199 | US10543183, Compound 12 | US109459...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [3H]-24,25-epoxycholesterol from human LXRbeta/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analys... | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-alpha/oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50204072 (CHEMBL3945199 | US10543183, Compound 12 | US109459...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [3H]-24,25-epoxycholesterol from human LXRalpha/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analy... | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50204072 (CHEMBL3945199 | US10543183, Compound 12 | US109459...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human LXR-alpha expressed in African green monkey CV1 cells measured after 18 to 20 hrs in presence of LXR pan agonist 1-(2,4-... | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50204072 (CHEMBL3945199 | US10543183, Compound 12 | US109459...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at LXR-beta in human HeLa cells assessed as induction of ABCA1 by beta-galactosidase/luciferase reporter gene assay | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50204072 (CHEMBL3945199 | US10543183, Compound 12 | US109459...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at LXR-beta in human whole blood assessed as ABCG1 gene induction by measuring ABCA1 mRNA level after 4 hrs by SYBR-Green dye-based ... | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50204072 (CHEMBL3945199 | US10543183, Compound 12 | US109459...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at human LXR-alpha expressed in African green monkey CV1 cells measured after 18 to 20 hrs by luciferase reporter gene assay | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50204072 (CHEMBL3945199 | US10543183, Compound 12 | US109459...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at LXR-beta in human whole blood assessed as ABCA1 gene induction by measuring ABCA1 mRNA level after 4 hrs by SYBR-Green dye-based ... | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pregnane X receptor (Homo sapiens (Human)) | BDBM50204072 (CHEMBL3945199 | US10543183, Compound 12 | US109459...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at human PXR expressed in human HepG2 cells assessed as induction of CYP3A4 measured after 24 hrs by AlamarBlue dye-based luciferase... | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50204072 (CHEMBL3945199 | US10543183, Compound 12 | US109459...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
The Rockefeller University US Patent | Assay Description As used herein, reference to the activity of an LXR agonist at LXRα and LXRβ refer to the activity as measured using the ligand sensing ass... | US Patent US10543183 (2020) BindingDB Entry DOI: 10.7270/Q2JW8H8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50204072 (CHEMBL3945199 | US10543183, Compound 12 | US109459...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
The Rockefeller University US Patent | Assay Description As used herein, reference to the activity of an LXR agonist at LXRα and LXRβ refer to the activity as measured using the ligand sensing ass... | US Patent US10543183 (2020) BindingDB Entry DOI: 10.7270/Q2JW8H8C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50204072 (CHEMBL3945199 | US10543183, Compound 12 | US109459...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
The Rockefeller University US Patent | Assay Description As used herein, reference to the activity of an LXR agonist at LXRα and LXRβ refer to the activity as measured using the ligand sensing ass... | US Patent US10945978 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50204072 (CHEMBL3945199 | US10543183, Compound 12 | US109459...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at human LXR-beta expressed in African green monkey CV1 cells measured after 18 to 20 hrs by luciferase reporter gene assay | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50204072 (CHEMBL3945199 | US10543183, Compound 12 | US109459...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
The Rockefeller University US Patent | Assay Description As used herein, reference to the activity of an LXR agonist at LXRα and LXRβ refer to the activity as measured using the ligand sensing ass... | US Patent US10945978 (2021) | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||