

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CNC(=O)Oc1ccc2CC3C4CCCCC4(CCN3CC3CCC3)c2c1
InChI Key: InChIKey=NHTWPKUCTUYFEM-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50204455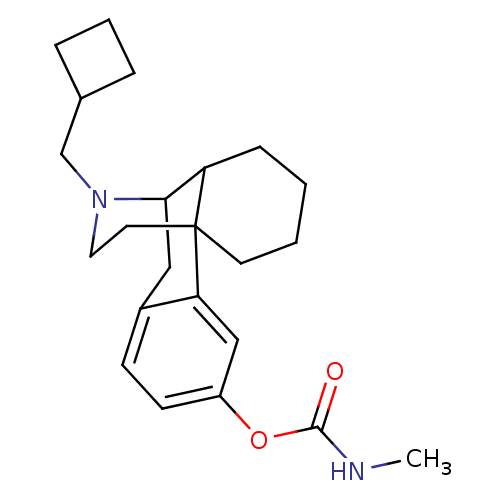 (CHEMBL242046 | MCL-435) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human kappa opioid receptors expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 1508-11 (2007) Article DOI: 10.1016/j.bmcl.2007.01.013 BindingDB Entry DOI: 10.7270/Q2WS8V2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50204455 (CHEMBL242046 | MCL-435) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptors expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 1508-11 (2007) Article DOI: 10.1016/j.bmcl.2007.01.013 BindingDB Entry DOI: 10.7270/Q2WS8V2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50204455 (CHEMBL242046 | MCL-435) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptors expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 1508-11 (2007) Article DOI: 10.1016/j.bmcl.2007.01.013 BindingDB Entry DOI: 10.7270/Q2WS8V2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50204455 (CHEMBL242046 | MCL-435) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as maximal stimulation of [35S]GTP-gamma-S binding | Bioorg Med Chem Lett 17: 1508-11 (2007) Article DOI: 10.1016/j.bmcl.2007.01.013 BindingDB Entry DOI: 10.7270/Q2WS8V2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50204455 (CHEMBL242046 | MCL-435) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at huma kappa opioid receptor expressed in CHO cells assessed as U50488-stimulated of [35S]GTP-gamma-S binding | Bioorg Med Chem Lett 17: 1508-11 (2007) Article DOI: 10.1016/j.bmcl.2007.01.013 BindingDB Entry DOI: 10.7270/Q2WS8V2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||