

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50206908 (+/-)-1-O-(4-cyanomethylphenyl)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol-3-yl)methylphenyl]-2-O-tetradecylglycerol::CHEMBL373870
SMILES: CCCCCCCCCCCCCCOC(COCc1ccc(cc1)C#N)COc1ccc(Cc2nc(=O)o[nH]2)cc1
InChI Key: InChIKey=AJHQQNUVUCZRJN-UHFFFAOYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Secretory phospholipase A2, group V (sPLA2-V) (Homo sapiens (Human)) | BDBM50206908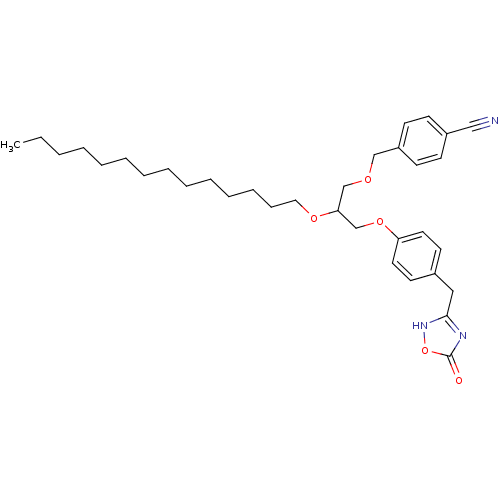 ((+/-)-1-O-(4-cyanomethylphenyl)-3-O-[4-(4,5-dihydr...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot Curated by ChEMBL | Assay Description Inhibition of human group V PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay | J Med Chem 50: 1618-26 (2007) Article DOI: 10.1021/jm060082n BindingDB Entry DOI: 10.7270/Q2TQ6174 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50206908 ((+/-)-1-O-(4-cyanomethylphenyl)-3-O-[4-(4,5-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot Curated by ChEMBL | Assay Description Inhibition of human group IIA PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay | J Med Chem 50: 1618-26 (2007) Article DOI: 10.1021/jm060082n BindingDB Entry DOI: 10.7270/Q2TQ6174 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, major isoenzyme (Sus scrofa (pig)) | BDBM50206908 ((+/-)-1-O-(4-cyanomethylphenyl)-3-O-[4-(4,5-dihydr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot Curated by ChEMBL | Assay Description Inhibition of pig group IB PLA2 by fluorimetric assay | J Med Chem 50: 1618-26 (2007) Article DOI: 10.1021/jm060082n BindingDB Entry DOI: 10.7270/Q2TQ6174 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 (Homo sapiens (Human)) | BDBM50206908 ((+/-)-1-O-(4-cyanomethylphenyl)-3-O-[4-(4,5-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot Curated by ChEMBL | Assay Description Inhibition of human group IB PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay | J Med Chem 50: 1618-26 (2007) Article DOI: 10.1021/jm060082n BindingDB Entry DOI: 10.7270/Q2TQ6174 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50206908 ((+/-)-1-O-(4-cyanomethylphenyl)-3-O-[4-(4,5-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot Curated by ChEMBL | Assay Description Inhibition of human group IIA PLA2 by fluorimetric assay | J Med Chem 50: 1618-26 (2007) Article DOI: 10.1021/jm060082n BindingDB Entry DOI: 10.7270/Q2TQ6174 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group X secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50206908 ((+/-)-1-O-(4-cyanomethylphenyl)-3-O-[4-(4,5-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot Curated by ChEMBL | Assay Description Inhibition of human group X PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay | J Med Chem 50: 1618-26 (2007) Article DOI: 10.1021/jm060082n BindingDB Entry DOI: 10.7270/Q2TQ6174 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||