

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50208940 5-bromo-pyridin-3-yl furan-2-carboxylate::5-bromopyridin-3-yl furan-2-carboxylate::CHEMBL222234::jm5b01461, Compound 121
SMILES: Brc1cncc(OC(=O)c2ccco2)c1
InChI Key: InChIKey=HMICIXHTRIFAET-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Papain-like protease (PLpro) (Human SARS coronavirus (SARS-CoV)) | BDBM50208940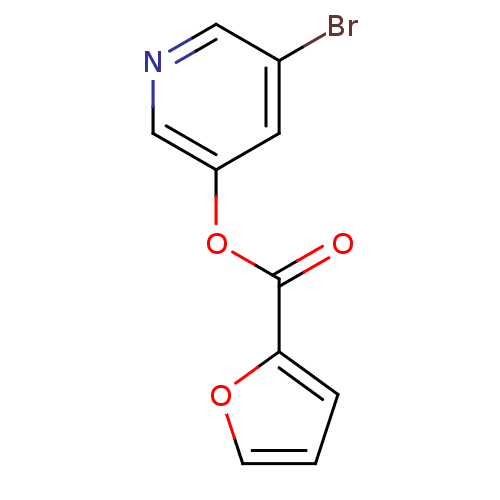 (5-bromo-pyridin-3-yl furan-2-carboxylate | 5-bromo...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3C-like protease | J Med Chem 50: 1850-64 (2007) Article DOI: 10.1021/jm061425k BindingDB Entry DOI: 10.7270/Q2RN37HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3C-like proteinase (3CL-PRO) (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50208940 (5-bromo-pyridin-3-yl furan-2-carboxylate | 5-bromo...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3C-like proteinase (3CL-PRO) (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50208940 (5-bromo-pyridin-3-yl furan-2-carboxylate | 5-bromo...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn | Assay Description This is a review article. | J Med Chem 59: 6595-628 (2016) Article DOI: 10.1021/acs.jmedchem.5b01461 BindingDB Entry DOI: 10.7270/Q2PK0JH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 16) | BDBM50208940 (5-bromo-pyridin-3-yl furan-2-carboxylate | 5-bromo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of HRV-16 protease 3C expressed in Escherichia coli BL21(DE3) by FRET assay | Bioorg Med Chem Lett 19: 3632-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.114 BindingDB Entry DOI: 10.7270/Q2542NHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||