

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50209974 1-((3S,4S)-4-(((S)-3-(4-fluorobenzyl)piperidin-1-yl)methyl)-1-acetylpiperidin-3-yl)-3-(5-acetyl-4-methylthiazol-2-yl)urea::CHEMBL249854
SMILES: CC(=O)N1CC[C@@H](CN2CCC[C@@H](Cc3ccc(F)cc3)C2)[C@@H](C1)NC(=O)Nc1nc(C)c(s1)C(C)=O
InChI Key: InChIKey=ZFRLEPMXXGKKBN-WPFOTENUSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50209974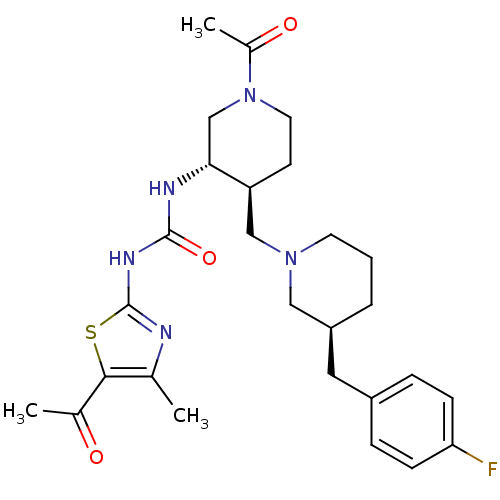 (1-((3S,4S)-4-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to dopamine transporter | Bioorg Med Chem Lett 17: 2992-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.065 BindingDB Entry DOI: 10.7270/Q2V987SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50209974 (1-((3S,4S)-4-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to norepinephrine transporter | Bioorg Med Chem Lett 17: 2992-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.065 BindingDB Entry DOI: 10.7270/Q2V987SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50209974 (1-((3S,4S)-4-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to serotonin transporter | Bioorg Med Chem Lett 17: 2992-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.065 BindingDB Entry DOI: 10.7270/Q2V987SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209974 (1-((3S,4S)-4-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at CCR3 assessed as eotaxin-induced chemotaxis in human eosinophils | Bioorg Med Chem Lett 17: 2992-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.065 BindingDB Entry DOI: 10.7270/Q2V987SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209974 (1-((3S,4S)-4-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at CCR3 in human eosinophils assessed as eotaxin-induced calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 17: 2992-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.065 BindingDB Entry DOI: 10.7270/Q2V987SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Mus musculus) | BDBM50209974 (1-((3S,4S)-4-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]eotaxin from BALB/c mouse CCR3 expressed in CHO cells after 30 mins | Bioorg Med Chem Lett 17: 2992-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.065 BindingDB Entry DOI: 10.7270/Q2V987SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50209974 (1-((3S,4S)-4-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of dopamine D2 receptor | Bioorg Med Chem Lett 17: 2992-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.065 BindingDB Entry DOI: 10.7270/Q2V987SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209974 (1-((3S,4S)-4-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]eotaxin from human CCR3 expressed in CHO cells after 30 mins | Bioorg Med Chem Lett 17: 2992-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.065 BindingDB Entry DOI: 10.7270/Q2V987SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Mus musculus) | BDBM50209974 (1-((3S,4S)-4-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at CCR3 assessed as eotaxin-induced chemotaxis in BALB/c mouse eosinophils | Bioorg Med Chem Lett 17: 2992-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.065 BindingDB Entry DOI: 10.7270/Q2V987SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50209974 (1-((3S,4S)-4-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of hERG | Bioorg Med Chem Lett 17: 2992-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.065 BindingDB Entry DOI: 10.7270/Q2V987SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50209974 (1-((3S,4S)-4-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of 5HT2A receptor | Bioorg Med Chem Lett 17: 2992-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.065 BindingDB Entry DOI: 10.7270/Q2V987SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50209974 (1-((3S,4S)-4-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2D6 | Bioorg Med Chem Lett 17: 2992-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.065 BindingDB Entry DOI: 10.7270/Q2V987SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||