

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50210730 4-((R)-1-((R)-6-(5-chloro-2-methoxybenzyl)-2,5-dioxo-1,4-diazepane-4-carboxamido)propyl)-2-aminobenzoic acid::CHEMBL245930
SMILES: CC[C@@H](NC(=O)N1CC(=O)NC[C@@H](Cc2cc(Cl)ccc2OC)C1=O)c1ccc(C(O)=O)c(N)c1
InChI Key: InChIKey=ZQCPJAZKZATEMD-DNVCBOLYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin G (Homo sapiens (Human)) | BDBM50210730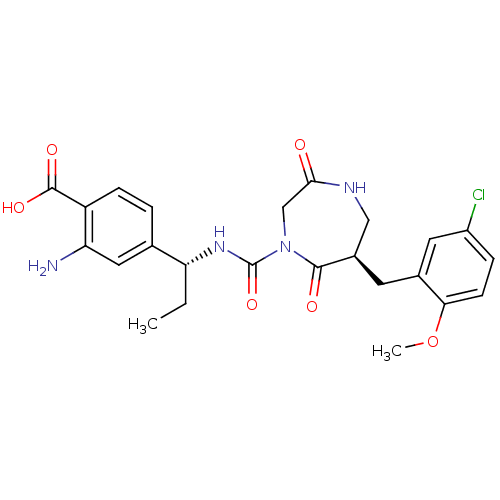 (4-((R)-1-((R)-6-(5-chloro-2-methoxybenzyl)-2,5-dio...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human cathepsin G | Bioorg Med Chem Lett 17: 3435-9 (2007) Article DOI: 10.1016/j.bmcl.2007.03.085 BindingDB Entry DOI: 10.7270/Q2MK6CKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50210730 (4-((R)-1-((R)-6-(5-chloro-2-methoxybenzyl)-2,5-dio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant chymase | Bioorg Med Chem Lett 17: 3435-9 (2007) Article DOI: 10.1016/j.bmcl.2007.03.085 BindingDB Entry DOI: 10.7270/Q2MK6CKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50210730 (4-((R)-1-((R)-6-(5-chloro-2-methoxybenzyl)-2,5-dio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase using MeOSuc-Ala-Ala-Pro-Val-pNA as substrate preincubated for 10 mins followed by substrate addition measure... | Bioorg Med Chem 21: 4233-49 (2013) Article DOI: 10.1016/j.bmc.2013.04.079 BindingDB Entry DOI: 10.7270/Q2CF9RHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50210730 (4-((R)-1-((R)-6-(5-chloro-2-methoxybenzyl)-2,5-dio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human chymase pre-incubated for 10 mins before Suc-Ala-Ala-Pro-Phe-MCA substrate addition and measured after 10 mins by flu... | Bioorg Med Chem Lett 28: 188-192 (2018) Article DOI: 10.1016/j.bmcl.2017.11.031 BindingDB Entry DOI: 10.7270/Q2T72M14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50210730 (4-((R)-1-((R)-6-(5-chloro-2-methoxybenzyl)-2,5-dio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant chymase using Suc-Ala-Ala-Pro-Phe-MCA as substrate assessed as AMC formation preincubated for 10 mins followed by sub... | Bioorg Med Chem 21: 4233-49 (2013) Article DOI: 10.1016/j.bmc.2013.04.079 BindingDB Entry DOI: 10.7270/Q2CF9RHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50210730 (4-((R)-1-((R)-6-(5-chloro-2-methoxybenzyl)-2,5-dio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a |
Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to recombinant human chymase assessed as dissociation constant at 6 to 1000 nM by SPR assay | Bioorg Med Chem Lett 28: 188-192 (2018) Article DOI: 10.1016/j.bmcl.2017.11.031 BindingDB Entry DOI: 10.7270/Q2T72M14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50210730 (4-((R)-1-((R)-6-(5-chloro-2-methoxybenzyl)-2,5-dio...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human neutrophil cathepsin G using Boc-Gln-Ala-Arg-MCA as substrate preincubated for 10 mins followed by substrate addition measured af... | Bioorg Med Chem 21: 4233-49 (2013) Article DOI: 10.1016/j.bmc.2013.04.079 BindingDB Entry DOI: 10.7270/Q2CF9RHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||