

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50213114 (S,S)-(+)-fenoterol::CHEMBL389390::US10617654, Compound (S,S)-1::US9492405, (S,S)-1::US9492405, 31
SMILES: C[C@@H](Cc1ccc(O)cc1)NC[C@@H](O)c1cc(O)cc(O)c1
InChI Key: InChIKey=LSLYOANBFKQKPT-APPDUMDISA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50213114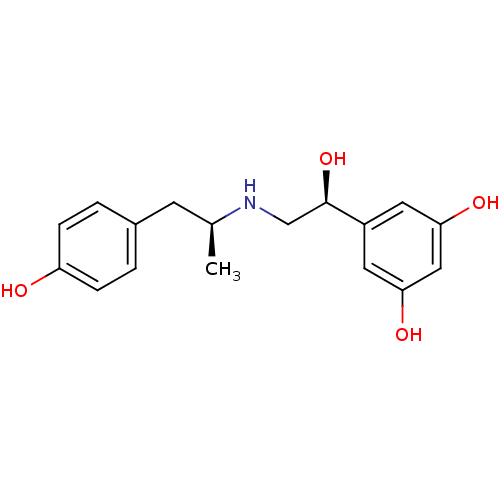 ((S,S)-(+)-fenoterol | CHEMBL389390 | US10617654, C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lublin Curated by ChEMBL | Assay Description Displacement of [3H]-(R,R')-methoxyfenoterol from human beta2 adrenergic receptor expressed in HEK cells by liquid scintillation counting analysis | Bioorg Med Chem 22: 234-46 (2014) Article DOI: 10.1016/j.bmc.2013.11.030 BindingDB Entry DOI: 10.7270/Q2251N5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50213114 ((S,S)-(+)-fenoterol | CHEMBL389390 | US10617654, C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lublin Curated by ChEMBL | Assay Description Displacement of [3H]CGP12177 from human beta-2 adrenergic receptor expressed in HEK293 cells | J Med Chem 50: 2903-15 (2007) Article DOI: 10.1021/jm070030d BindingDB Entry DOI: 10.7270/Q2R2112T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50213114 ((S,S)-(+)-fenoterol | CHEMBL389390 | US10617654, C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 2.77E+4 | -5.77 | n/a | n/a | n/a | n/a | n/a | 7.8 | 4 |
The United States of America, as represented by the Secretary, Department of Health and Human Services; SRI International US Patent | Assay Description Beta1-AR binding was done on rat cortical membrane following a previously described procedure (Beer et al., Biochem. Pharmacol. 37: 1145-1151, 1988).... | US Patent US9492405 (2016) BindingDB Entry DOI: 10.7270/Q2CJ8CBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50213114 ((S,S)-(+)-fenoterol | CHEMBL389390 | US10617654, C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | >1.00E+5 | >-5.07 | n/a | n/a | n/a | n/a | n/a | 7.8 | 4 |
The United States of America, as represented by the Secretary, Department of Health and Human Services; SRI International US Patent | Assay Description Beta1-AR binding was done on rat cortical membrane following a previously described procedure (Beer et al., Biochem. Pharmacol. 37: 1145-1151, 1988).... | US Patent US9492405 (2016) BindingDB Entry DOI: 10.7270/Q2CJ8CBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50213114 ((S,S)-(+)-fenoterol | CHEMBL389390 | US10617654, C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lublin Curated by ChEMBL | Assay Description Displacement of [3H]CGP-12177 from beta-1 adrenergic receptor in Sprague-Dawley rat cortical membrane | J Med Chem 50: 2903-15 (2007) Article DOI: 10.1021/jm070030d BindingDB Entry DOI: 10.7270/Q2R2112T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50213114 ((S,S)-(+)-fenoterol | CHEMBL389390 | US10617654, C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 2.77E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The USA, as represented by the Secretary, Department of Health and Human Services; SRI International US Patent | Assay Description HEK 293 cells stability transfected with cDNA encoding human β2-AR (provided by Dr. Brian Kobilka, Stanford Medical Center, Palo Alto, Calif.) w... | US Patent US10617654 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic recepto (Mus musculus) | BDBM50213114 ((S,S)-(+)-fenoterol | CHEMBL389390 | US10617654, C...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | >1.00E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The USA, as represented by the Secretary, Department of Health and Human Services; SRI International US Patent | Assay Description β1-AR binding was done on rat cortical membrane following a previously described procedure (Beer et al., Biochem. Pharmacol. 37: 1145-1151, 1988... | US Patent US10617654 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50213114 ((S,S)-(+)-fenoterol | CHEMBL389390 | US10617654, C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 2.75E+4 | n/a | n/a | n/a | 7.7 | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services; SRI International US Patent | Assay Description HEK 293 cells stability transfected with cDNA encoding human beta2-AR (provided by Dr. Brian Kobilka, Stanford Medical Center, Palo Alto, Calif.) wer... | US Patent US9492405 (2016) BindingDB Entry DOI: 10.7270/Q2CJ8CBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50213114 ((S,S)-(+)-fenoterol | CHEMBL389390 | US10617654, C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 184 | n/a | n/a | n/a | n/a | n/a | 37 |
The United States of America, as represented by the Secretary, Department of Health and Human Services; SRI International US Patent | Assay Description To measure beta2-AR mediated inhibition of mitogenesis, HEK-beta2-AR, 1321N1 or U87MG cells were seeded in a 96-well plate at approximately 5,000 c... | US Patent US9492405 (2016) BindingDB Entry DOI: 10.7270/Q2CJ8CBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50213114 ((S,S)-(+)-fenoterol | CHEMBL389390 | US10617654, C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 2.75E+4 | n/a | n/a | n/a | n/a | n/a |
The USA, as represented by the Secretary, Department of Health and Human Services; SRI International US Patent | Assay Description HEK 293 cells stability transfected with cDNA encoding human β2-AR (provided by Dr. Brian Kobilka, Stanford Medical Center, Palo Alto, Calif.) w... | US Patent US10617654 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||