

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50224179 1-{2-[(1S)-(3-dimethylaminopropionyl)amino-2-methylpropyl]-4-methylphenyl}-4-[(2R)-methyl-3-(2-fluoro-4-chlorophenyl)propionyl]piperazine::CHEMBL391056::N-((S)-1-(2-(4-((R)-3-(4-chloro-2-fluorophenyl)-2-methylpropanoyl)piperazin-1-yl)-5-methylphenyl)-2-methylpropyl)-3-(dimethylamino)propanamide
SMILES: CC(C)[C@H](NC(=O)CCN(C)C)c1cc(C)ccc1N1CCN(CC1)C(=O)[C@H](C)Cc1ccc(Cl)cc1F
InChI Key: InChIKey=NIDQERYOGAPSJL-MNNSJKJDSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50224179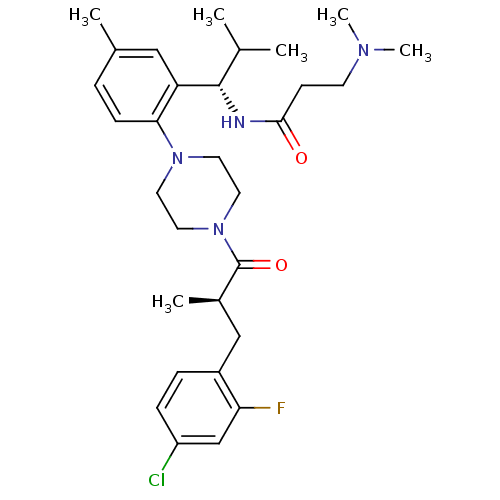 (1-{2-[(1S)-(3-dimethylaminopropionyl)amino-2-methy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]NDP-MSH from human MC4R expressed in HEK293 cells | J Med Chem 50: 5249-52 (2007) Article DOI: 10.1021/jm070806a BindingDB Entry DOI: 10.7270/Q29C6X6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50224179 (1-{2-[(1S)-(3-dimethylaminopropionyl)amino-2-methy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]NDP-MSH from human MC4R expressed in HEK293 cells | Bioorg Med Chem 16: 5606-18 (2008) Article DOI: 10.1016/j.bmc.2008.03.072 BindingDB Entry DOI: 10.7270/Q2TX3F54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50224179 (1-{2-[(1S)-(3-dimethylaminopropionyl)amino-2-methy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human MC4R expressed in CHO cells assessed as inhibition of alpha-MSH-induced cAMP production by ELISA | Bioorg Med Chem 16: 5606-18 (2008) Article DOI: 10.1016/j.bmc.2008.03.072 BindingDB Entry DOI: 10.7270/Q2TX3F54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50224179 (1-{2-[(1S)-(3-dimethylaminopropionyl)amino-2-methy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Antagonist activity at MC4R assessed as inhibition of alpha-MSH-stimulated cAMP release | J Med Chem 50: 5249-52 (2007) Article DOI: 10.1021/jm070806a BindingDB Entry DOI: 10.7270/Q29C6X6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50224179 (1-{2-[(1S)-(3-dimethylaminopropionyl)amino-2-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Med Chem 50: 5249-52 (2007) Article DOI: 10.1021/jm070806a BindingDB Entry DOI: 10.7270/Q29C6X6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||