

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50225415 CHEMBL237830::N,N'-di-2-pyridinyl-1,4-benzenedimethanamine::US9205085, MSX- 121::WZ-811
SMILES: C(Nc1ccccn1)c1ccc(CNc2ccccn2)cc1
InChI Key: InChIKey=KBVFRXIGQQRMEF-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 4 (CXCR4/SDF-1) (Rattus norvegicus (Rat)) | BDBM50225415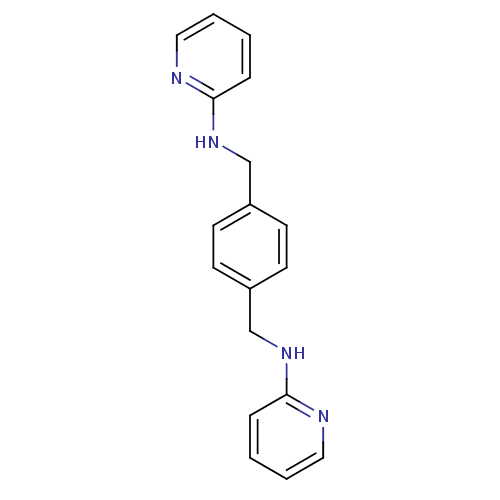 (CHEMBL237830 | N,N'-di-2-pyridinyl-1,4-benzenedime...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University US Patent | Assay Description A synthetic 14-mer peptide, TN14003, was previously reported to block both SDF-1/CXCR4 mediated invasion in vitro and metastasis in vivo with a high ... | US Patent US9205085 (2015) BindingDB Entry DOI: 10.7270/Q2FF3R5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50225415 (CHEMBL237830 | N,N'-di-2-pyridinyl-1,4-benzenedime...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Calabria Curated by ChEMBL | Assay Description Binding affinity to CXCR4 in human MDA-MB-231 cells preincubated for 15 mins followed by biotinylated TN41003 addition measured after 30 mins by rhod... | Eur J Med Chem 139: 519-530 (2017) Article DOI: 10.1016/j.ejmech.2017.08.027 BindingDB Entry DOI: 10.7270/Q21J9D9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (CXCR4) (Homo sapiens (Human)) | BDBM50225415 (CHEMBL237830 | N,N'-di-2-pyridinyl-1,4-benzenedime...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 expressed in human U87 cells expressing CD4 assessed as inhibition of CXCL12-induced cAMP production pretreated fo... | J Med Chem 53: 8556-68 (2010) Article DOI: 10.1021/jm100786g BindingDB Entry DOI: 10.7270/Q2PK0GH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (CXCR4) (Homo sapiens (Human)) | BDBM50225415 (CHEMBL237830 | N,N'-di-2-pyridinyl-1,4-benzenedime...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of biotinylated TN14003 from CXCR4 in MDA-MB-231 cells | J Med Chem 50: 5655-64 (2007) Article DOI: 10.1021/jm070679i BindingDB Entry DOI: 10.7270/Q2X066RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||