

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50241244 (E)-((2R,3S,4S,5R,6S)-6-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-3-yloxy)-3,4,5-trihydroxy-tetrahydro-2H-pyran-2-yl)methyl 3-(4-hydroxyphenyl)acrylate::CHEMBL266564::CHEMBL499705::Tiliroside::kaempferol 3-O-(6'' ''-O-E-p-coumaroyl)-beta-D-glucopyranoside::kaempferol-3-beta-D-(6-O-trans-p-coumaroyl)glucopyranoside::trans-Tiliroside
SMILES: O[C@@H]1[C@@H](COC(=O)C=Cc2ccc(O)cc2)O[C@@H](Oc2c(O)c3c(cc(O)cc3=O)oc2-c2ccc(O)cc2)[C@H](O)[C@H]1O
InChI Key: InChIKey=HZLHIOUWCDTOOF-FIZCXTQCSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50241244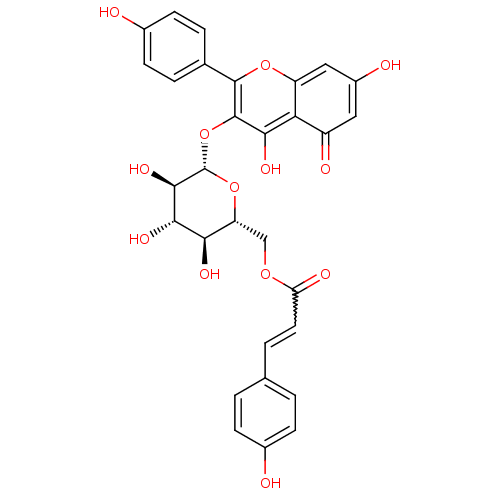 ((E)-((2R,3S,4S,5R,6S)-6-(5,7-dihydroxy-2-(4-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.60 | -11.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Aristotle University of Thessaloniki Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 7 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa... | Bioorg Med Chem 23: 7219-25 (2015) BindingDB Entry DOI: 10.7270/Q2862J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50241244 ((E)-((2R,3S,4S,5R,6S)-6-(5,7-dihydroxy-2-(4-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 134 | -9.37 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Aristotle University of Thessaloniki Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration ass... | Bioorg Med Chem 23: 7219-25 (2015) BindingDB Entry DOI: 10.7270/Q2862J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50241244 ((E)-((2R,3S,4S,5R,6S)-6-(5,7-dihydroxy-2-(4-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.47E+3 | -7.17 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Aristotle University of Thessaloniki Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa... | Bioorg Med Chem 23: 7219-25 (2015) BindingDB Entry DOI: 10.7270/Q2862J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50241244 ((E)-((2R,3S,4S,5R,6S)-6-(5,7-dihydroxy-2-(4-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | >-6.82 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Aristotle University of Thessaloniki Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa... | Bioorg Med Chem 23: 7219-25 (2015) BindingDB Entry DOI: 10.7270/Q2862J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50241244 ((E)-((2R,3S,4S,5R,6S)-6-(5,7-dihydroxy-2-(4-hydrox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | >-6.82 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Aristotle University of Thessaloniki Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 1 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa... | Bioorg Med Chem 23: 7219-25 (2015) BindingDB Entry DOI: 10.7270/Q2862J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50241244 ((E)-((2R,3S,4S,5R,6S)-6-(5,7-dihydroxy-2-(4-hydrox...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <3.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Inhibition of HIV1 RT | J Nat Prod 54: 143-54 Article DOI: 10.1021/np50073a012 BindingDB Entry DOI: 10.7270/Q2NK3HTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50241244 ((E)-((2R,3S,4S,5R,6S)-6-(5,7-dihydroxy-2-(4-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 | J Nat Prod 67: 1839-41 (2004) Article DOI: 10.1021/np0400104 BindingDB Entry DOI: 10.7270/Q2571BRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50241244 ((E)-((2R,3S,4S,5R,6S)-6-(5,7-dihydroxy-2-(4-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 | J Nat Prod 67: 1839-41 (2004) Article DOI: 10.1021/np0400104 BindingDB Entry DOI: 10.7270/Q2571BRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glyceraldehyde-3-phosphate dehydrogenase, glycosomal (Trypanosoma cruzi) | BDBM50241244 ((E)-((2R,3S,4S,5R,6S)-6-(5,7-dihydroxy-2-(4-hydrox...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi recombinant glycosomal GAPDH expressed in Escherichia coli by spectrophotometry | Bioorg Med Chem 17: 2476-82 (2009) Article DOI: 10.1016/j.bmc.2009.01.079 BindingDB Entry DOI: 10.7270/Q2BC40FV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50241244 ((E)-((2R,3S,4S,5R,6S)-6-(5,7-dihydroxy-2-(4-hydrox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase assessed as decrease in uric acid production by spectrophotometry | J Nat Prod 61: 71-6 (1998) Article DOI: 10.1021/np970237h BindingDB Entry DOI: 10.7270/Q29C6Z93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||