

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50242451 CHEMBL510801::hemopressin
SMILES: CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1)C(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O
InChI Key: InChIKey=DUTLYPZZJJBEAJ-QISMNGAHSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50242451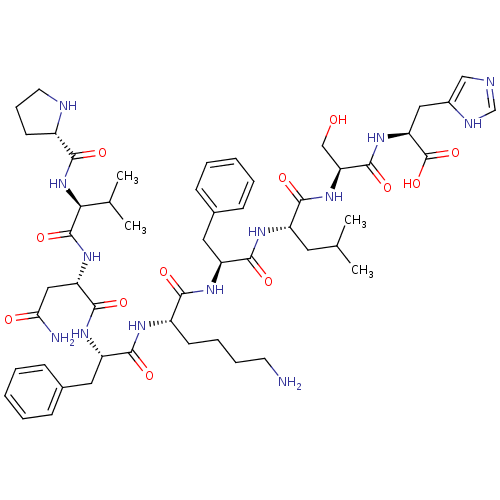 (CHEMBL510801 | hemopressin) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biological Research Centre of the Hungarian Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]JWH-018 from CB1R/CB2R in Wistar rat brain membranes after 60 mins by liquid scintillation analysis | Eur J Med Chem 178: 571-588 (2019) Article DOI: 10.1016/j.ejmech.2019.05.037 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50242451 (CHEMBL510801 | hemopressin) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a |
Proteimax Biotechnology Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from Long-Evans rat striatal membrane CB1 receptor | Proc Natl Acad Sci U S A 104: 20588-93 (2007) Article DOI: 10.1073/pnas.0706980105 BindingDB Entry DOI: 10.7270/Q23N234W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||