

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50244165 (1R,2S,3R,5Z,7E)-17-{(1R)-1-[(2-ethyl-2-hydroxybutyl)sulfanyl]ethyl}-2-(2-hydroxyethoxy)-9,10-secoestra-5,7,16-triene-1,3-diol::(20R)-1-alpha,25-Dihydroxy-2-alpha-(2-hydroxyethoxy)-16-ene-22-thia-26,27-dimethyl-19,24-dinorvitamin D3::(20R)-1-alpha-25-dihydroxy-2-beta-(2-hydroxyethoxy)-16-ene-22-thia-26,27-dimethyl-19,24-dinorvitamin D3::CHEMBL459034::CHEMBL514116
SMILES: CCC(O)(CC)CS[C@H](C)C1=CC[C@H]2\C(CCC[C@]12C)=C\C=C1C[C@@H](O)C(OCCO)[C@H](O)C1
InChI Key: InChIKey=DUIYWCMQVXJTIP-PKTKNRMZSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin D receptor (Mus musculus) | BDBM50244165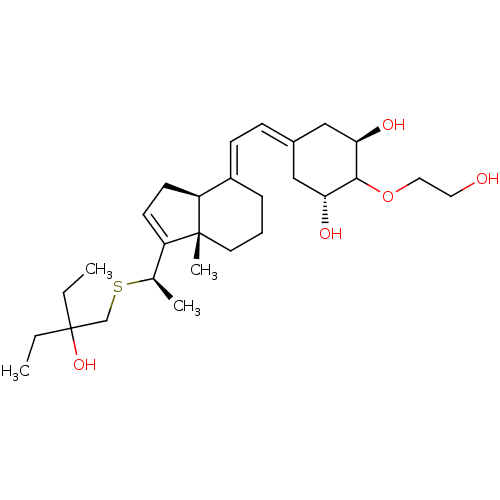 ((1R,2S,3R,5Z,7E)-17-{(1R)-1-[(2-ethyl-2-hydroxybut...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.115 | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Increase in mouse VDR-mediated transcriptional activity in african green monkey COS7 cells after 24 hrs by luciferase assay | Bioorg Med Chem 16: 6949-64 (2008) Article DOI: 10.1016/j.bmc.2008.05.043 BindingDB Entry DOI: 10.7270/Q2Z60NWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D receptor (Mus musculus) | BDBM50244165 ((1R,2S,3R,5Z,7E)-17-{(1R)-1-[(2-ethyl-2-hydroxybut...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0240 | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Increase in mouse VDR-mediated transcriptional activity in african green monkey COS7 cells after 24 hrs by luciferase assay | Bioorg Med Chem 16: 6949-64 (2008) Article DOI: 10.1016/j.bmc.2008.05.043 BindingDB Entry DOI: 10.7270/Q2Z60NWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||