

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50245647 (1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS)-1,2,6a,6b,9,9,12a-heptamethyl-10-oxo-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylic acid::3-Oxours-12-en-28-oic Acid::3-oxo-12-ursen-28-oic acid::3-oxo-urs-12-en-28-oic acid::CHEMBL487887
SMILES: C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CCC(=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O
InChI Key: InChIKey=MUCRYNWJQNHDJH-OADIDDRXSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 19A1 (Homo sapiens (Human)) | BDBM50245647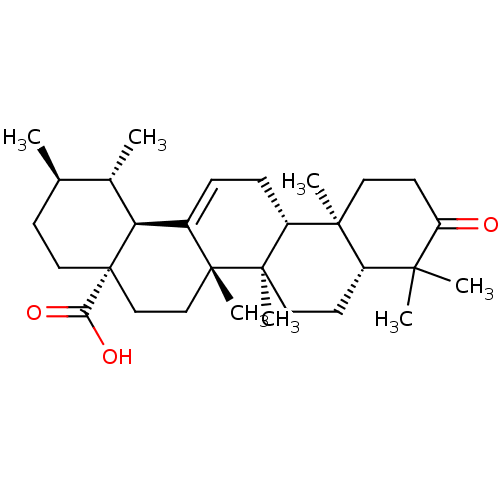 ((1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS)-1,2,6a,6b,9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS) Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes assessed as tritiated water release after 15 mins using [1beta, 3H]androstenedione as substrate... | Eur J Med Chem 43: 1865-77 (2008) Article DOI: 10.1016/j.ejmech.2007.11.021 BindingDB Entry DOI: 10.7270/Q2ZW1KPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50245647 ((1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS)-1,2,6a,6b,9...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of cathepsin D (unknown origin) using hemoglobin as substrate after 30 min by spectrophotometric analysis | Citation and Details Article DOI: 10.1007/s00044-012-0397-z BindingDB Entry DOI: 10.7270/Q29026PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50245647 ((1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS)-1,2,6a,6b,9...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase A assessed as release of phosphate from glucose-1-phosphate after 25 mins by microplate reader bas... | Eur J Med Chem 46: 2011-21 (2011) Article DOI: 10.1016/j.ejmech.2011.02.053 BindingDB Entry DOI: 10.7270/Q2DB826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||