

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50246594 (+/-)-7-((1-hydroxy-3-(methylthio)propan-2-ylamino)methyl)-3H-pyrrolo[3,2-d]pyrimidin-4(5H)-one::CHEMBL462398::US9290501, MT-SerMe-ImmH
SMILES: CSCC(CO)NCc1c[nH]c2c1nc[nH]c2=O
InChI Key: InChIKey=CHAYSRLWZZJIPO-UHFFFAOYSA-N
Data: 4 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S-methyl-5'-thioinosine phosphorylase (Pseudomonas aeruginosa) | BDBM50246594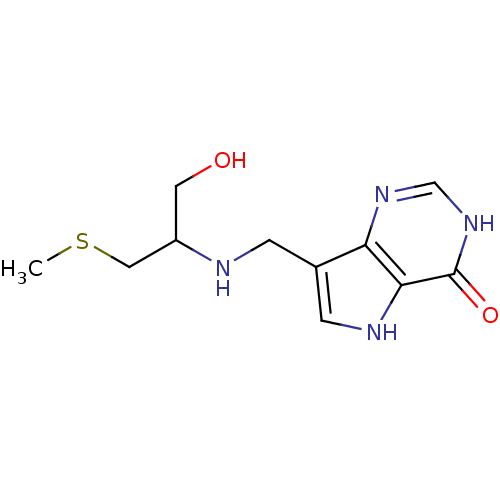 ((+/-)-7-((1-hydroxy-3-(methylthio)propan-2-ylamino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0960 | -13.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Albert Einstein College of Medicine, Inc.; Victoria Link Limited US Patent | Assay Description Assays for slow-onset inhibitors were carried out by adding 1 nM PaMTIP into reaction mixtures at 25 °C. containing 100 mM Hepes, pH 7.4, 100 mM ... | US Patent US9290501 (2016) BindingDB Entry DOI: 10.7270/Q2639NKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioinosine phosphorylase (Pseudomonas aeruginosa) | BDBM50246594 ((+/-)-7-((1-hydroxy-3-(methylthio)propan-2-ylamino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0960 | -13.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Albert Einstein College of Medicine, Inc.; Victoria Link Limited US Patent | Assay Description Assays for slow-onset inhibitors were carried out by adding 1 nM PaMTIP into reaction mixtures at 25 °C. containing 100 mM Hepes, pH 7.4, 100 mM ... | US Patent US9290501 (2016) BindingDB Entry DOI: 10.7270/Q2639NKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioinosine phosphorylase (Pseudomonas aeruginosa) | BDBM50246594 ((+/-)-7-((1-hydroxy-3-(methylthio)propan-2-ylamino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.720 | -12.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Albert Einstein College of Medicine, Inc.; Victoria Link Limited US Patent | Assay Description Assays for slow-onset inhibitors were carried out by adding 1 nM PaMTIP into reaction mixtures at 25 °C. containing 100 mM Hepes, pH 7.4, 100 mM ... | US Patent US9290501 (2016) BindingDB Entry DOI: 10.7270/Q2639NKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50246594 ((+/-)-7-((1-hydroxy-3-(methylthio)propan-2-ylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine of Yeshiva University Curated by ChEMBL | Assay Description Initial binding affinity to wild type human PNP | Bioorg Med Chem Lett 18: 5900-3 (2008) Article DOI: 10.1016/j.bmcl.2008.08.047 BindingDB Entry DOI: 10.7270/Q2SX6F4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||