 Found 15 hits for monomerid = 50247012
Found 15 hits for monomerid = 50247012 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
11-beta-hydroxysteroid dehydrogenase
(Homo sapiens (Human)) | BDBM50247012
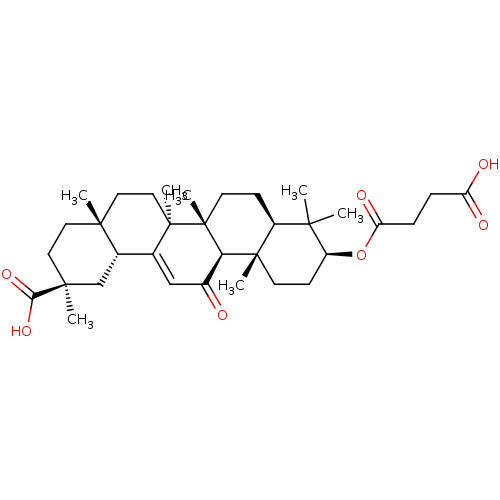
(3beta-O-Succinyl-18-beta-glycyrrhetinic acid | Car...)Show SMILES CC1(C)[C@H](CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O)OC(=O)CCC(O)=O |r,t:18| Show InChI InChI=1S/C34H50O7/c1-29(2)23-10-13-34(7)27(32(23,5)12-11-24(29)41-26(38)9-8-25(36)37)22(35)18-20-21-19-31(4,28(39)40)15-14-30(21,3)16-17-33(20,34)6/h18,21,23-24,27H,8-17,19H2,1-7H3,(H,36,37)(H,39,40)/t21-,23-,24-,27+,30+,31-,32-,33+,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50247012

(3beta-O-Succinyl-18-beta-glycyrrhetinic acid | Car...)Show SMILES CC1(C)[C@H](CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O)OC(=O)CCC(O)=O |r,t:18| Show InChI InChI=1S/C34H50O7/c1-29(2)23-10-13-34(7)27(32(23,5)12-11-24(29)41-26(38)9-8-25(36)37)22(35)18-20-21-19-31(4,28(39)40)15-14-30(21,3)16-17-33(20,34)6/h18,21,23-24,27H,8-17,19H2,1-7H3,(H,36,37)(H,39,40)/t21-,23-,24-,27+,30+,31-,32-,33+,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 243 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
|
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50247012

(3beta-O-Succinyl-18-beta-glycyrrhetinic acid | Car...)Show SMILES CC1(C)[C@H](CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O)OC(=O)CCC(O)=O |r,t:18| Show InChI InChI=1S/C34H50O7/c1-29(2)23-10-13-34(7)27(32(23,5)12-11-24(29)41-26(38)9-8-25(36)37)22(35)18-20-21-19-31(4,28(39)40)15-14-30(21,3)16-17-33(20,34)6/h18,21,23-24,27H,8-17,19H2,1-7H3,(H,36,37)(H,39,40)/t21-,23-,24-,27+,30+,31-,32-,33+,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50247012

(3beta-O-Succinyl-18-beta-glycyrrhetinic acid | Car...)Show SMILES CC1(C)[C@H](CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O)OC(=O)CCC(O)=O |r,t:18| Show InChI InChI=1S/C34H50O7/c1-29(2)23-10-13-34(7)27(32(23,5)12-11-24(29)41-26(38)9-8-25(36)37)22(35)18-20-21-19-31(4,28(39)40)15-14-30(21,3)16-17-33(20,34)6/h18,21,23-24,27H,8-17,19H2,1-7H3,(H,36,37)(H,39,40)/t21-,23-,24-,27+,30+,31-,32-,33+,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum
Curated by ChEMBL
| Assay Description
Ability to convert [3H]cortisol to the tritium labeled cortisone in the presence of human 11 beta hydroxysteroid dehydrogenase type 2 |
J Med Chem 45: 3813-5 (2002)
BindingDB Entry DOI: 10.7270/Q2S46SPW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50247012

(3beta-O-Succinyl-18-beta-glycyrrhetinic acid | Car...)Show SMILES CC1(C)[C@H](CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O)OC(=O)CCC(O)=O |r,t:18| Show InChI InChI=1S/C34H50O7/c1-29(2)23-10-13-34(7)27(32(23,5)12-11-24(29)41-26(38)9-8-25(36)37)22(35)18-20-21-19-31(4,28(39)40)15-14-30(21,3)16-17-33(20,34)6/h18,21,23-24,27H,8-17,19H2,1-7H3,(H,36,37)(H,39,40)/t21-,23-,24-,27+,30+,31-,32-,33+,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against mouse 11 beta hydroxysteroid dehydrogenase type 1 using scintillation proximity assay (SPA). |
J Med Chem 45: 3813-5 (2002)
BindingDB Entry DOI: 10.7270/Q2S46SPW |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50247012

(3beta-O-Succinyl-18-beta-glycyrrhetinic acid | Car...)Show SMILES CC1(C)[C@H](CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O)OC(=O)CCC(O)=O |r,t:18| Show InChI InChI=1S/C34H50O7/c1-29(2)23-10-13-34(7)27(32(23,5)12-11-24(29)41-26(38)9-8-25(36)37)22(35)18-20-21-19-31(4,28(39)40)15-14-30(21,3)16-17-33(20,34)6/h18,21,23-24,27H,8-17,19H2,1-7H3,(H,36,37)(H,39,40)/t21-,23-,24-,27+,30+,31-,32-,33+,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human 11 beta hydroxysteroid dehydrogenase type 1 using scintillation proximity assay (SPA) |
J Med Chem 45: 3813-5 (2002)
BindingDB Entry DOI: 10.7270/Q2S46SPW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-Hydroxysteroid Dehydrogenase 2 (11-beta-HSD2)
(Mus musculus (mouse)) | BDBM50247012

(3beta-O-Succinyl-18-beta-glycyrrhetinic acid | Car...)Show SMILES CC1(C)[C@H](CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O)OC(=O)CCC(O)=O |r,t:18| Show InChI InChI=1S/C34H50O7/c1-29(2)23-10-13-34(7)27(32(23,5)12-11-24(29)41-26(38)9-8-25(36)37)22(35)18-20-21-19-31(4,28(39)40)15-14-30(21,3)16-17-33(20,34)6/h18,21,23-24,27H,8-17,19H2,1-7H3,(H,36,37)(H,39,40)/t21-,23-,24-,27+,30+,31-,32-,33+,34+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mouse microsomal 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol by scintillation proximi... |
Bioorg Med Chem Lett 19: 4455-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.033
BindingDB Entry DOI: 10.7270/Q2VT1T1D |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50247012

(3beta-O-Succinyl-18-beta-glycyrrhetinic acid | Car...)Show SMILES CC1(C)[C@H](CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O)OC(=O)CCC(O)=O |r,t:18| Show InChI InChI=1S/C34H50O7/c1-29(2)23-10-13-34(7)27(32(23,5)12-11-24(29)41-26(38)9-8-25(36)37)22(35)18-20-21-19-31(4,28(39)40)15-14-30(21,3)16-17-33(20,34)6/h18,21,23-24,27H,8-17,19H2,1-7H3,(H,36,37)(H,39,40)/t21-,23-,24-,27+,30+,31-,32-,33+,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells using cortisone as substrate after 3 hrs by HTRF assay |
ACS Med Chem Lett 3: 88-93 (2012)
Article DOI: 10.1021/ml200226x
BindingDB Entry DOI: 10.7270/Q2TH8NR0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-Hydroxysteroid Dehydrogenase 2 (11-beta-HSD2)
(Mus musculus (mouse)) | BDBM50247012

(3beta-O-Succinyl-18-beta-glycyrrhetinic acid | Car...)Show SMILES CC1(C)[C@H](CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O)OC(=O)CCC(O)=O |r,t:18| Show InChI InChI=1S/C34H50O7/c1-29(2)23-10-13-34(7)27(32(23,5)12-11-24(29)41-26(38)9-8-25(36)37)22(35)18-20-21-19-31(4,28(39)40)15-14-30(21,3)16-17-33(20,34)6/h18,21,23-24,27H,8-17,19H2,1-7H3,(H,36,37)(H,39,40)/t21-,23-,24-,27+,30+,31-,32-,33+,34+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [3H]cortisone into [3H]cortisol by scintillation proximity assay |
J Nat Prod 75: 599-604 (2012)
Article DOI: 10.1021/np200831c
BindingDB Entry DOI: 10.7270/Q27M090Q |
More data for this
Ligand-Target Pair | |
11-beta-Hydroxysteroid Dehydrogenase 2 (11-beta-HSD2)
(Mus musculus (mouse)) | BDBM50247012

(3beta-O-Succinyl-18-beta-glycyrrhetinic acid | Car...)Show SMILES CC1(C)[C@H](CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O)OC(=O)CCC(O)=O |r,t:18| Show InChI InChI=1S/C34H50O7/c1-29(2)23-10-13-34(7)27(32(23,5)12-11-24(29)41-26(38)9-8-25(36)37)22(35)18-20-21-19-31(4,28(39)40)15-14-30(21,3)16-17-33(20,34)6/h18,21,23-24,27H,8-17,19H2,1-7H3,(H,36,37)(H,39,40)/t21-,23-,24-,27+,30+,31-,32-,33+,34+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 82 | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD2 expressed in HEK293 cells assessed as [3H]cortisol production after 60 mins by SPA |
Bioorg Med Chem Lett 22: 2748-52 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.095
BindingDB Entry DOI: 10.7270/Q22F7PGX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase
(Homo sapiens (Human)) | BDBM50247012

(3beta-O-Succinyl-18-beta-glycyrrhetinic acid | Car...)Show SMILES CC1(C)[C@H](CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O)OC(=O)CCC(O)=O |r,t:18| Show InChI InChI=1S/C34H50O7/c1-29(2)23-10-13-34(7)27(32(23,5)12-11-24(29)41-26(38)9-8-25(36)37)22(35)18-20-21-19-31(4,28(39)40)15-14-30(21,3)16-17-33(20,34)6/h18,21,23-24,27H,8-17,19H2,1-7H3,(H,36,37)(H,39,40)/t21-,23-,24-,27+,30+,31-,32-,33+,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase
(Homo sapiens (Human)) | BDBM50247012

(3beta-O-Succinyl-18-beta-glycyrrhetinic acid | Car...)Show SMILES CC1(C)[C@H](CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O)OC(=O)CCC(O)=O |r,t:18| Show InChI InChI=1S/C34H50O7/c1-29(2)23-10-13-34(7)27(32(23,5)12-11-24(29)41-26(38)9-8-25(36)37)22(35)18-20-21-19-31(4,28(39)40)15-14-30(21,3)16-17-33(20,34)6/h18,21,23-24,27H,8-17,19H2,1-7H3,(H,36,37)(H,39,40)/t21-,23-,24-,27+,30+,31-,32-,33+,34+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD2 (unknown origin) expressed in HEK293 cells using cortisone as substrate measured after 2 hrs in presence of NAD+ by HTRF as... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase
(Homo sapiens (Human)) | BDBM50247012

(3beta-O-Succinyl-18-beta-glycyrrhetinic acid | Car...)Show SMILES CC1(C)[C@H](CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O)OC(=O)CCC(O)=O |r,t:18| Show InChI InChI=1S/C34H50O7/c1-29(2)23-10-13-34(7)27(32(23,5)12-11-24(29)41-26(38)9-8-25(36)37)22(35)18-20-21-19-31(4,28(39)40)15-14-30(21,3)16-17-33(20,34)6/h18,21,23-24,27H,8-17,19H2,1-7H3,(H,36,37)(H,39,40)/t21-,23-,24-,27+,30+,31-,32-,33+,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50247012

(3beta-O-Succinyl-18-beta-glycyrrhetinic acid | Car...)Show SMILES CC1(C)[C@H](CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O)OC(=O)CCC(O)=O |r,t:18| Show InChI InChI=1S/C34H50O7/c1-29(2)23-10-13-34(7)27(32(23,5)12-11-24(29)41-26(38)9-8-25(36)37)22(35)18-20-21-19-31(4,28(39)40)15-14-30(21,3)16-17-33(20,34)6/h18,21,23-24,27H,8-17,19H2,1-7H3,(H,36,37)(H,39,40)/t21-,23-,24-,27+,30+,31-,32-,33+,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes by HTRF cortisol assay |
Bioorg Med Chem Lett 20: 1065-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.035
BindingDB Entry DOI: 10.7270/Q26M36XD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50247012

(3beta-O-Succinyl-18-beta-glycyrrhetinic acid | Car...)Show SMILES CC1(C)[C@H](CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O)OC(=O)CCC(O)=O |r,t:18| Show InChI InChI=1S/C34H50O7/c1-29(2)23-10-13-34(7)27(32(23,5)12-11-24(29)41-26(38)9-8-25(36)37)22(35)18-20-21-19-31(4,28(39)40)15-14-30(21,3)16-17-33(20,34)6/h18,21,23-24,27H,8-17,19H2,1-7H3,(H,36,37)(H,39,40)/t21-,23-,24-,27+,30+,31-,32-,33+,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data