 Found 11 hits for monomerid = 50248510
Found 11 hits for monomerid = 50248510 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50248510
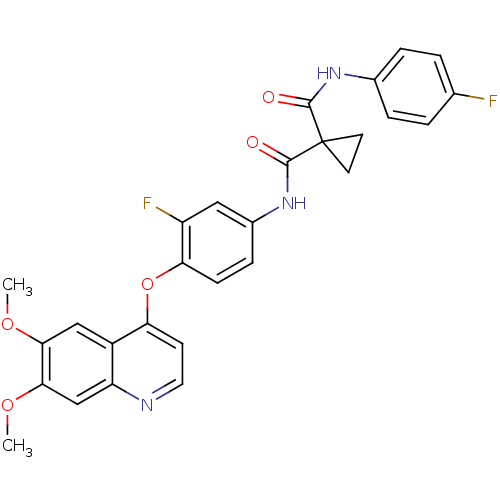
(CHEMBL489326 | N-(4-(6,7-dimethoxyquinolin-4-yloxy...)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)c2cc1OC Show InChI InChI=1S/C28H23F2N3O5/c1-36-24-14-19-21(15-25(24)37-2)31-12-9-22(19)38-23-8-7-18(13-20(23)30)33-27(35)28(10-11-28)26(34)32-17-5-3-16(29)4-6-17/h3-9,12-15H,10-11H2,1-2H3,(H,32,34)(H,33,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) |
Bioorg Med Chem Lett 19: 1323-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.068
BindingDB Entry DOI: 10.7270/Q23F4PHJ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50248510

(CHEMBL489326 | N-(4-(6,7-dimethoxyquinolin-4-yloxy...)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)c2cc1OC Show InChI InChI=1S/C28H23F2N3O5/c1-36-24-14-19-21(15-25(24)37-2)31-12-9-22(19)38-23-8-7-18(13-20(23)30)33-27(35)28(10-11-28)26(34)32-17-5-3-16(29)4-6-17/h3-9,12-15H,10-11H2,1-2H3,(H,32,34)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) |
Bioorg Med Chem Lett 19: 1323-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.068
BindingDB Entry DOI: 10.7270/Q23F4PHJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50248510

(CHEMBL489326 | N-(4-(6,7-dimethoxyquinolin-4-yloxy...)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)c2cc1OC Show InChI InChI=1S/C28H23F2N3O5/c1-36-24-14-19-21(15-25(24)37-2)31-12-9-22(19)38-23-8-7-18(13-20(23)30)33-27(35)28(10-11-28)26(34)32-17-5-3-16(29)4-6-17/h3-9,12-15H,10-11H2,1-2H3,(H,32,34)(H,33,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of AXL in human MDA-MB-231 cells assessed as receptor phosphorylation after 1 to 3 hrs |
J Med Chem 59: 3593-608 (2016)
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50248510

(CHEMBL489326 | N-(4-(6,7-dimethoxyquinolin-4-yloxy...)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)c2cc1OC Show InChI InChI=1S/C28H23F2N3O5/c1-36-24-14-19-21(15-25(24)37-2)31-12-9-22(19)38-23-8-7-18(13-20(23)30)33-27(35)28(10-11-28)26(34)32-17-5-3-16(29)4-6-17/h3-9,12-15H,10-11H2,1-2H3,(H,32,34)(H,33,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length AXL using poly (Glu,Tyr) as substrate by AlphaScreen assay |
J Med Chem 59: 3593-608 (2016)
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50248510

(CHEMBL489326 | N-(4-(6,7-dimethoxyquinolin-4-yloxy...)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)c2cc1OC Show InChI InChI=1S/C28H23F2N3O5/c1-36-24-14-19-21(15-25(24)37-2)31-12-9-22(19)38-23-8-7-18(13-20(23)30)33-27(35)28(10-11-28)26(34)32-17-5-3-16(29)4-6-17/h3-9,12-15H,10-11H2,1-2H3,(H,32,34)(H,33,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of MET in human MDA-MB-231 cells assessed as receptor phosphorylation after 1 to 3 hrs |
J Med Chem 59: 3593-608 (2016)
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50248510

(CHEMBL489326 | N-(4-(6,7-dimethoxyquinolin-4-yloxy...)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)c2cc1OC Show InChI InChI=1S/C28H23F2N3O5/c1-36-24-14-19-21(15-25(24)37-2)31-12-9-22(19)38-23-8-7-18(13-20(23)30)33-27(35)28(10-11-28)26(34)32-17-5-3-16(29)4-6-17/h3-9,12-15H,10-11H2,1-2H3,(H,32,34)(H,33,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged c-Met expressed in Sf9 cells |
Bioorg Med Chem Lett 19: 6552-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.040
BindingDB Entry DOI: 10.7270/Q2VM4D6R |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50248510

(CHEMBL489326 | N-(4-(6,7-dimethoxyquinolin-4-yloxy...)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)c2cc1OC Show InChI InChI=1S/C28H23F2N3O5/c1-36-24-14-19-21(15-25(24)37-2)31-12-9-22(19)38-23-8-7-18(13-20(23)30)33-27(35)28(10-11-28)26(34)32-17-5-3-16(29)4-6-17/h3-9,12-15H,10-11H2,1-2H3,(H,32,34)(H,33,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MET using poly (Glu,Tyr) as substrate by AlphaScreen assay |
J Med Chem 59: 3593-608 (2016)
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50248510

(CHEMBL489326 | N-(4-(6,7-dimethoxyquinolin-4-yloxy...)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)c2cc1OC Show InChI InChI=1S/C28H23F2N3O5/c1-36-24-14-19-21(15-25(24)37-2)31-12-9-22(19)38-23-8-7-18(13-20(23)30)33-27(35)28(10-11-28)26(34)32-17-5-3-16(29)4-6-17/h3-9,12-15H,10-11H2,1-2H3,(H,32,34)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length VEGFR2 using poly (Glu,Tyr) as substrate by AlphaScreen assay |
J Med Chem 59: 3593-608 (2016)
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50248510

(CHEMBL489326 | N-(4-(6,7-dimethoxyquinolin-4-yloxy...)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)c2cc1OC Show InChI InChI=1S/C28H23F2N3O5/c1-36-24-14-19-21(15-25(24)37-2)31-12-9-22(19)38-23-8-7-18(13-20(23)30)33-27(35)28(10-11-28)26(34)32-17-5-3-16(29)4-6-17/h3-9,12-15H,10-11H2,1-2H3,(H,32,34)(H,33,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human c-MET |
Eur J Med Chem 46: 3675-80 (2011)
Article DOI: 10.1016/j.ejmech.2011.05.031
BindingDB Entry DOI: 10.7270/Q2891676 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50248510

(CHEMBL489326 | N-(4-(6,7-dimethoxyquinolin-4-yloxy...)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)c2cc1OC Show InChI InChI=1S/C28H23F2N3O5/c1-36-24-14-19-21(15-25(24)37-2)31-12-9-22(19)38-23-8-7-18(13-20(23)30)33-27(35)28(10-11-28)26(34)32-17-5-3-16(29)4-6-17/h3-9,12-15H,10-11H2,1-2H3,(H,32,34)(H,33,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) |
Eur J Med Chem 64: 62-73 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.001
BindingDB Entry DOI: 10.7270/Q27082SZ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50248510

(CHEMBL489326 | N-(4-(6,7-dimethoxyquinolin-4-yloxy...)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)c2cc1OC Show InChI InChI=1S/C28H23F2N3O5/c1-36-24-14-19-21(15-25(24)37-2)31-12-9-22(19)38-23-8-7-18(13-20(23)30)33-27(35)28(10-11-28)26(34)32-17-5-3-16(29)4-6-17/h3-9,12-15H,10-11H2,1-2H3,(H,32,34)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 in human MDA-MB-231 cells assessed as receptor phosphorylation after 1 to 3 hrs |
J Med Chem 59: 3593-608 (2016)
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data