

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50249566 CHEMBL4085254
SMILES: CC1NC(NCCC[C@H](N)C(O)=O)=NC1=O
InChI Key: InChIKey=KGQMQNPFMOBJCY-GDVGLLTNSA-N
Data: 1 Kd
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249566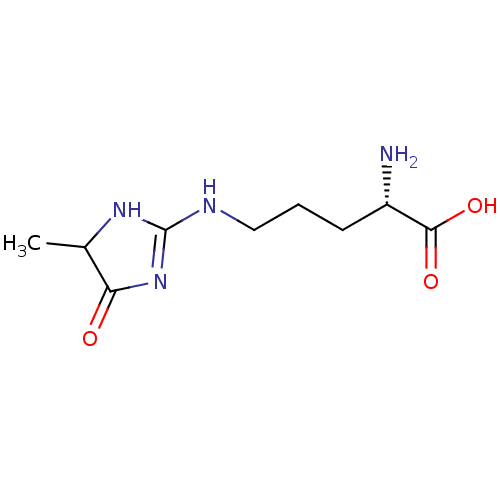 (CHEMBL4085254) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a |
King's College London Curated by ChEMBL | Assay Description Binding affinity to human C-terminal His-tagged RAGE domain V (24 to 125 residues) expressed in Escherichia coli BL21(DE3) by tryptophan fluorescence... | J Med Chem 60: 7213-7232 (2017) Article DOI: 10.1021/acs.jmedchem.7b00058 BindingDB Entry DOI: 10.7270/Q2KD21BP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||