

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50250665 CHEMBL4076264::US10961200, Compound 425
SMILES: OC(=O)c1csc(n1)-n1nc(cc1O)-c1ccc(F)cc1
InChI Key: InChIKey=DTOIPNVKTYNDGF-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50250665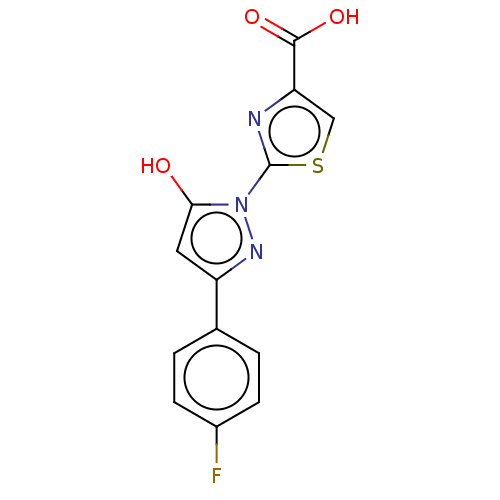 (CHEMBL4076264 | US10961200, Compound 425) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human liver LDHA using sodium pyruvate as substrate after 5 mins in presence of NAPDH and in absence of EDTA by diaphorase/resazurin ba... | J Med Chem 60: 9184-9204 (2017) Article DOI: 10.1021/acs.jmedchem.7b00941 BindingDB Entry DOI: 10.7270/Q2DJ5J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50250665 (CHEMBL4076264 | US10961200, Compound 425) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
THE UNITED STATES OF AMERICA, AS REPRESENTED BY THE SECRETARY, DEPARTMENT OF HEALTH AND HUMAN SERVICES; VANDERBILT UNIVERSITY; THE UAB RESEARCH FOUNDATION; THE TRUSTEES OF THE UNIVERSITY OF PENNSYLVANIA US Patent | Assay Description Test compounds were placed in a Greiner Bio-One (Monroe, N.C.) 1536-well black solid bottom assay plate. 200 millimolar (mM) Tris HCl, pH 7.4, 100 mi... | US Patent US10961200 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50250665 (CHEMBL4076264 | US10961200, Compound 425) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human liver LDHA using sodium pyruvate as substrate after 5 mins in presence of NAPDH and in absence of EDTA by diaphorase/resazurin ba... | J Med Chem 60: 9184-9204 (2017) Article DOI: 10.1021/acs.jmedchem.7b00941 BindingDB Entry DOI: 10.7270/Q2DJ5J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||