

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50252096 (+/-)-N-(1-(3-bromo-4-(1-methylpyrrolidin-3-yloxy)benzyl)pyrrolidin-3-yl)-3,4-dichlorobenzamide::CHEMBL518301
SMILES: CN1CCC(C1)Oc1ccc(CN2CCC(C2)NC(=O)c2ccc(Cl)c(Cl)c2)cc1Br
InChI Key: InChIKey=XESAEBNNDALGPZ-UHFFFAOYSA-N
Data: 1 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urotensin II receptor (Homo sapiens (Human)) | BDBM50252096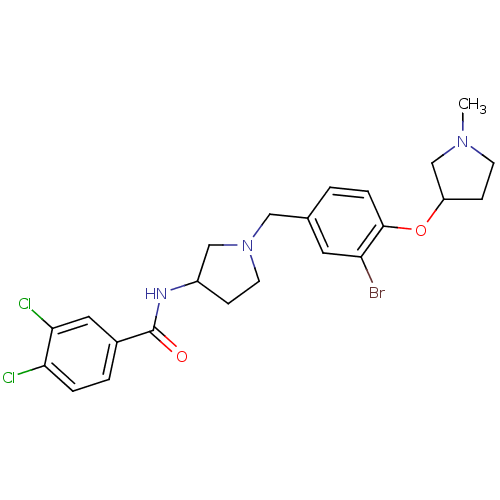 ((+/-)-N-(1-(3-bromo-4-(1-methylpyrrolidin-3-yloxy)...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant urotensin 2 receptor expressed in HEK293 cells assessed as inhibition of urotensin 2-induced calcium mobiliz... | Bioorg Med Chem Lett 18: 3950-4 (2008) Article DOI: 10.1016/j.bmcl.2008.06.019 BindingDB Entry DOI: 10.7270/Q2FT8KTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||