

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50253092 CHEMBL4062319
SMILES: Cc1nnc2sc(C(=O)NC3CN(C3)c3cccc(F)c3F)c(N)c2c1C
InChI Key: InChIKey=KWYCAGNWNMBWOL-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50253092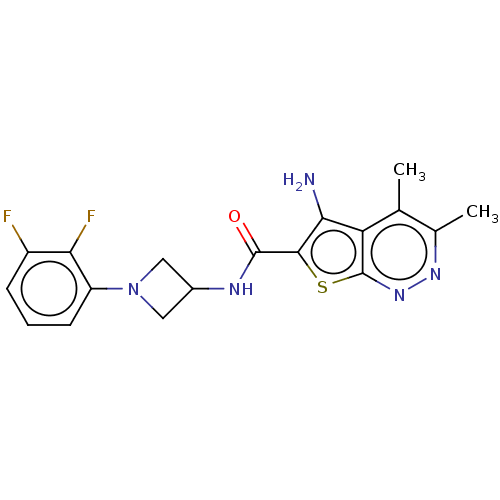 (CHEMBL4062319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Center for Neuroscience Drug Discovery, Vanderbilt University School of Medicine, Nashville, TN 37232, USA. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | Bioorg Med Chem Lett 27: 2990-2995 (2017) Article DOI: 10.1016/j.bmcl.2017.05.014 BindingDB Entry DOI: 10.7270/Q2MS3W6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50253092 (CHEMBL4062319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Center for Neuroscience Drug Discovery, Vanderbilt University School of Medicine, Nashville, TN 37232, USA. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | Bioorg Med Chem Lett 27: 2990-2995 (2017) Article DOI: 10.1016/j.bmcl.2017.05.014 BindingDB Entry DOI: 10.7270/Q2MS3W6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 and M4 (Homo sapiens (Human)) | BDBM50253092 (CHEMBL4062319) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Vanderbilt Center for Neuroscience Drug Discovery, Vanderbilt University School of Medicine, Nashville, TN 37232, USA. Curated by ChEMBL | Assay Description Positive allosteric modulation of human M4 receptor expressed in CHO cells co-expressing Gqi5 assessed as increase in acetylcholine-induced calcium m... | Bioorg Med Chem Lett 27: 2990-2995 (2017) Article DOI: 10.1016/j.bmcl.2017.05.014 BindingDB Entry DOI: 10.7270/Q2MS3W6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 and M4 (Homo sapiens (Human)) | BDBM50253092 (CHEMBL4062319) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Vanderbilt Center for Neuroscience Drug Discovery, Vanderbilt University School of Medicine, Nashville, TN 37232, USA. Curated by ChEMBL | Assay Description Positive allosteric modulation of human M4 receptor expressed in CHO cells co-expressing Gqi5 assessed as increase in acetylcholine-induced calcium m... | Bioorg Med Chem Lett 27: 2990-2995 (2017) Article DOI: 10.1016/j.bmcl.2017.05.014 BindingDB Entry DOI: 10.7270/Q2MS3W6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM50253092 (CHEMBL4062319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Center for Neuroscience Drug Discovery, Vanderbilt University School of Medicine, Nashville, TN 37232, USA. Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) | Bioorg Med Chem Lett 27: 2990-2995 (2017) Article DOI: 10.1016/j.bmcl.2017.05.014 BindingDB Entry DOI: 10.7270/Q2MS3W6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50253092 (CHEMBL4062319) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Center for Neuroscience Drug Discovery, Vanderbilt University School of Medicine, Nashville, TN 37232, USA. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | Bioorg Med Chem Lett 27: 2990-2995 (2017) Article DOI: 10.1016/j.bmcl.2017.05.014 BindingDB Entry DOI: 10.7270/Q2MS3W6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50253092 (CHEMBL4062319) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Center for Neuroscience Drug Discovery, Vanderbilt University School of Medicine, Nashville, TN 37232, USA. Curated by ChEMBL | Assay Description Displacement of [3H]BTCP from human recombinant dopamine transporter expressed in CHO cells | Bioorg Med Chem Lett 27: 2990-2995 (2017) Article DOI: 10.1016/j.bmcl.2017.05.014 BindingDB Entry DOI: 10.7270/Q2MS3W6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50253092 (CHEMBL4062319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Center for Neuroscience Drug Discovery, Vanderbilt University School of Medicine, Nashville, TN 37232, USA. Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) | Bioorg Med Chem Lett 27: 2990-2995 (2017) Article DOI: 10.1016/j.bmcl.2017.05.014 BindingDB Entry DOI: 10.7270/Q2MS3W6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||