

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50253803 2-(4-chloro-6-(2,3-dimethylphenylamino)pyrimidin-2-ylthio)-2-phenylacetic acid::CHEMBL461409
SMILES: Cc1cccc(Nc2cc(Cl)nc(SC(C(O)=O)c3ccccc3)n2)c1C
InChI Key: InChIKey=SABFOCWZUFNJNB-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase (cyclooxygenase) (Ovis aries (Sheep)) | BDBM50253803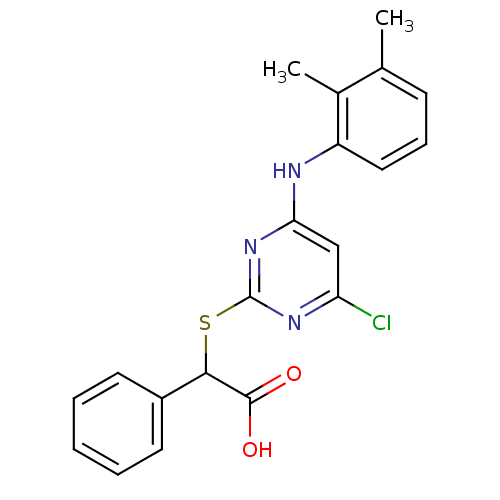 (2-(4-chloro-6-(2,3-dimethylphenylamino)pyrimidin-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of ovine COX1 | J Med Chem 51: 8068-76 (2008) Article DOI: 10.1021/jm801085s BindingDB Entry DOI: 10.7270/Q2639QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM50253803 (2-(4-chloro-6-(2,3-dimethylphenylamino)pyrimidin-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 | J Med Chem 51: 8068-76 (2008) Article DOI: 10.1021/jm801085s BindingDB Entry DOI: 10.7270/Q2639QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor (Homo sapiens (Human)) | BDBM50253803 (2-(4-chloro-6-(2,3-dimethylphenylamino)pyrimidin-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Activation of human Gal4-fused PPARgamma LBD transfected in HEK293T after 14 to 16 hrs by dual-glo luciferase reporter gene assay | J Med Chem 62: 2112-2126 (2019) Article DOI: 10.1021/acs.jmedchem.8b01848 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor (Homo sapiens (Human)) | BDBM50253803 (2-(4-chloro-6-(2,3-dimethylphenylamino)pyrimidin-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Activation of human Gal4-fused PPARgamma LBD transfected in HEK293T after 14 to 16 hrs by dual-glo luciferase reporter gene assay | J Med Chem 62: 2112-2126 (2019) Article DOI: 10.1021/acs.jmedchem.8b01848 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor (Homo sapiens (Human)) | BDBM50253803 (2-(4-chloro-6-(2,3-dimethylphenylamino)pyrimidin-2...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Activation of human Gal4-fused PPARalpha LBD transfected in HEK293T after 14 to 16 hrs by dual-glo luciferase reporter gene assay | J Med Chem 62: 2112-2126 (2019) Article DOI: 10.1021/acs.jmedchem.8b01848 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor (Homo sapiens (Human)) | BDBM50253803 (2-(4-chloro-6-(2,3-dimethylphenylamino)pyrimidin-2...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Activation of human Gal4-fused PPARalpha LBD transfected in HEK293T after 14 to 16 hrs by dual-glo luciferase reporter gene assay | J Med Chem 62: 2112-2126 (2019) Article DOI: 10.1021/acs.jmedchem.8b01848 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||