

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50258733 6,7-Dimethoxy-1-(3'-nitrophenyl)-3,4-dihydroisoquinoline-2(1H)-sulfonamide::CHEMBL468105
SMILES: COc1cc2CCN(C(c3cccc(c3)[N+]([O-])=O)c2cc1OC)S(N)(=O)=O
InChI Key: InChIKey=GRCLVIQEAYEYKM-UHFFFAOYSA-N
Data: 4 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50258733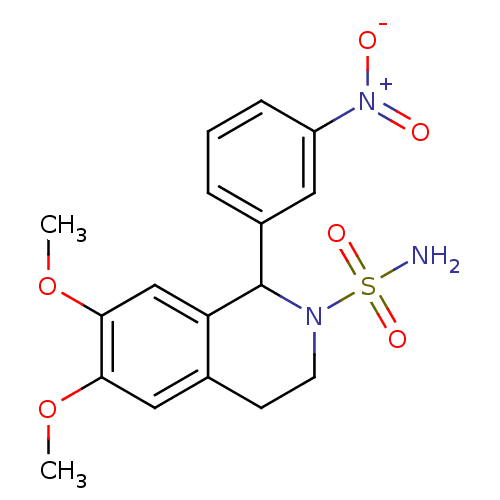 (6,7-Dimethoxy-1-(3'-nitrophenyl)-3,4-dihydroisoqui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Messina Curated by ChEMBL | Assay Description Inhibition of human recombinant full length carbonic anhydrase 2 by stopped flow CO2 hydration method | Bioorg Med Chem 17: 3659-64 (2009) Article DOI: 10.1016/j.bmc.2009.03.066 BindingDB Entry DOI: 10.7270/Q21R6RFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic Anhydrase XIV (Homo sapiens (Human)) | BDBM50258733 (6,7-Dimethoxy-1-(3'-nitrophenyl)-3,4-dihydroisoqui...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Messina Curated by ChEMBL | Assay Description Inhibition of human recombinant full length carbonic anhydrase 14 by stopped flow CO2 hydration method | Bioorg Med Chem 17: 3659-64 (2009) Article DOI: 10.1016/j.bmc.2009.03.066 BindingDB Entry DOI: 10.7270/Q21R6RFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50258733 (6,7-Dimethoxy-1-(3'-nitrophenyl)-3,4-dihydroisoqui...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Messina Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 9 catalytic domain by stopped flow CO2 hydration method | Bioorg Med Chem 17: 3659-64 (2009) Article DOI: 10.1016/j.bmc.2009.03.066 BindingDB Entry DOI: 10.7270/Q21R6RFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50258733 (6,7-Dimethoxy-1-(3'-nitrophenyl)-3,4-dihydroisoqui...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Messina Curated by ChEMBL | Assay Description Inhibition of human recombinant full length carbonic anhydrase 1 by stopped flow CO2 hydration method | Bioorg Med Chem 17: 3659-64 (2009) Article DOI: 10.1016/j.bmc.2009.03.066 BindingDB Entry DOI: 10.7270/Q21R6RFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||