

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50261400 (3R,6S,9S,12S,15R,18S,21S,24S)-24-Acetylamino-12-(4-amino-butyl)-6,18,21-tribenzyl-9-((R)-1-hydroxy-ethyl)-15-(1H-indol-3-ylmethyl)-5,8,11,14,17,20,23-heptaoxo-1,2-dithia-4,7,10,13,16,19,22-heptaaza-cyclopentacosane-3-carboxylic acid::CHEMBL503036
SMILES: C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CSS[C@@H](NC(=O)[C@H](Cc2ccccc2)NC1=O)C(O)=O)NC(C)=O
InChI Key: InChIKey=OIRQKWLKQYCEFH-UAYIBRAQSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50261400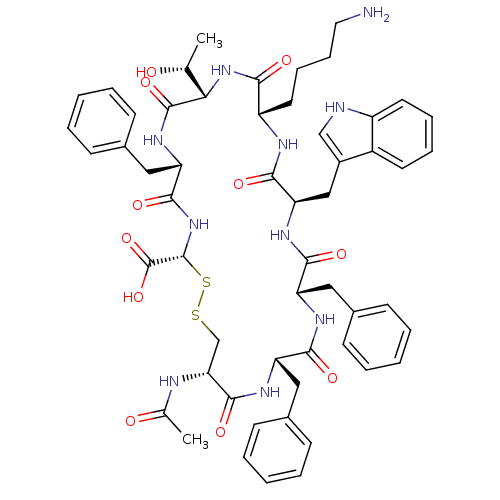 ((3R,6S,9S,12S,15R,18S,21S,24S)-24-Acetylamino-12-(...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Displacement of [125I]-[Leu8, DTrp22, Tyr25]SRIF-28 from human cloned sst4 receptor expressed in CCL39 cells | J Med Chem 51: 2668-75 (2008) Article DOI: 10.1021/jm701444y BindingDB Entry DOI: 10.7270/Q2CF9R1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50261400 ((3R,6S,9S,12S,15R,18S,21S,24S)-24-Acetylamino-12-(...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Displacement of [125I]-[Leu8, DTrp22, Tyr25]SRIF-28 from human cloned sst5 receptor expressed in CHOK1 cells | J Med Chem 51: 2668-75 (2008) Article DOI: 10.1021/jm701444y BindingDB Entry DOI: 10.7270/Q2CF9R1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50261400 ((3R,6S,9S,12S,15R,18S,21S,24S)-24-Acetylamino-12-(...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Displacement of [125I]-[Leu8, DTrp22, Tyr25]SRIF-28 from human cloned sst3 receptor expressed in CCL39 cells | J Med Chem 51: 2668-75 (2008) Article DOI: 10.1021/jm701444y BindingDB Entry DOI: 10.7270/Q2CF9R1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50261400 ((3R,6S,9S,12S,15R,18S,21S,24S)-24-Acetylamino-12-(...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Displacement of [125I]-[Leu8, DTrp22, Tyr25]SRIF-28 from human cloned sst2 receptor expressed in CCL39 cells | J Med Chem 51: 2668-75 (2008) Article DOI: 10.1021/jm701444y BindingDB Entry DOI: 10.7270/Q2CF9R1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 1 (Homo sapiens (Human)) | BDBM50261400 ((3R,6S,9S,12S,15R,18S,21S,24S)-24-Acetylamino-12-(...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Displacement of [125I]-[Leu8, DTrp22, Tyr25]SRIF28 from human cloned sst1 receptor | J Med Chem 51: 2668-75 (2008) Article DOI: 10.1021/jm701444y BindingDB Entry DOI: 10.7270/Q2CF9R1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||