

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50267991 (R)-2-(3-(3-(4-methoxybenzoyl)-2-methyl-6-(trifluoromethoxy)-1H-indol-1-yl)phenoxy)butanoic acid::CHEMBL490029
SMILES: CC[C@@H](Oc1cccc(c1)-n1c(C)c(C(=O)c2ccc(OC)cc2)c2ccc(OC(F)(F)F)cc12)C(O)=O
InChI Key: InChIKey=STWITCBWQHTJFJ-XMMPIXPASA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor alpha (PPAR alpha) (Homo sapiens (Human)) | BDBM50267991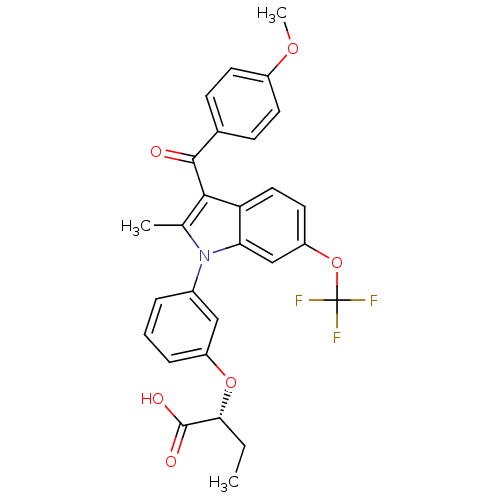 ((R)-2-(3-(3-(4-methoxybenzoyl)-2-methyl-6-(trifluo...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H2]nTZD3 from human recombinant GST-fused PPARalpha expressed in Escherichia coli by scintillation proximity assay | J Med Chem 52: 3846-54 (2009) Article DOI: 10.1021/jm900097m BindingDB Entry DOI: 10.7270/Q2GF0TDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor (Homo sapiens (Human)) | BDBM50267991 ((R)-2-(3-(3-(4-methoxybenzoyl)-2-methyl-6-(trifluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human recombinant PPARgamma expressed in COS1 cells coexpressing GAL4 assessed as transcriptional activity after 48 hrs by lucife... | J Med Chem 52: 3846-54 (2009) Article DOI: 10.1021/jm900097m BindingDB Entry DOI: 10.7270/Q2GF0TDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Canis familiaris) | BDBM50267991 ((R)-2-(3-(3-(4-methoxybenzoyl)-2-methyl-6-(trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 370 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at dog PPARalpha | J Med Chem 52: 3846-54 (2009) Article DOI: 10.1021/jm900097m BindingDB Entry DOI: 10.7270/Q2GF0TDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Mus musculus) | BDBM50267991 ((R)-2-(3-(3-(4-methoxybenzoyl)-2-methyl-6-(trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at mouse PPARalpha | J Med Chem 52: 3846-54 (2009) Article DOI: 10.1021/jm900097m BindingDB Entry DOI: 10.7270/Q2GF0TDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor (Homo sapiens (Human)) | BDBM50267991 ((R)-2-(3-(3-(4-methoxybenzoyl)-2-methyl-6-(trifluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H2]nTZD3 from human recombinant GST-fused PPARgamma expressed in Escherichia coli by scintillation proximity assay | J Med Chem 52: 3846-54 (2009) Article DOI: 10.1021/jm900097m BindingDB Entry DOI: 10.7270/Q2GF0TDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||