

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50269758 CHEMBL4103723
SMILES: O[C@]1(Cc2cc(on2)-c2cn[nH]c2)CC[C@H](Cc2ccc(F)cc2)CC1
InChI Key: InChIKey=OCOUQAJDGFNHSQ-GSXCWMCISA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor ionotropic, NMDA 2B (Mus musculus) | BDBM50269758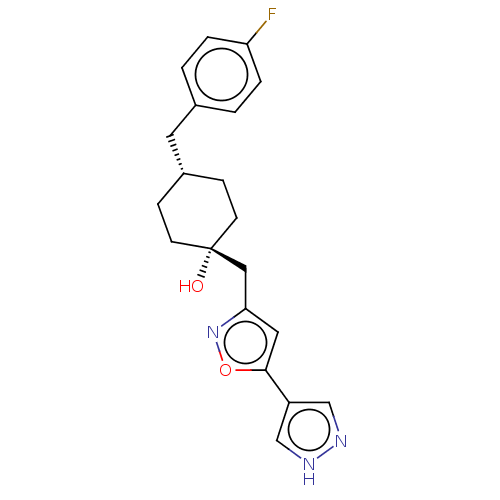 (CHEMBL4103723) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Research Laboratory, Shionogi& Co Ltd, 1-1 Futabacho, 3-chome, Toyonaka 561-0825, Japan. Electronic address: kousuke.anan@shionogi.co.jp. Curated by ChEMBL | Assay Description Antagonist activity at mouse NR2B expressed in Hek293 cells co-expressing mouse NR1 assessed as inhibition of glutamic acid/glycine-induced intracell... | Bioorg Med Chem Lett 27: 4194-4198 (2017) Article DOI: 10.1016/j.bmcl.2017.06.076 BindingDB Entry DOI: 10.7270/Q2VX0K0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50269758 (CHEMBL4103723) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Research Laboratory, Shionogi& Co Ltd, 1-1 Futabacho, 3-chome, Toyonaka 561-0825, Japan. Electronic address: kousuke.anan@shionogi.co.jp. Curated by ChEMBL | Assay Description Displacement of [3H]-ifenprodil from NR2B in Wistar rat brain membrane after 120 mins by liquid scintillation counting analysis | Bioorg Med Chem Lett 27: 4194-4198 (2017) Article DOI: 10.1016/j.bmcl.2017.06.076 BindingDB Entry DOI: 10.7270/Q2VX0K0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||