

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50271102 ((((2R,3S,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(hydroxy)phosphoryl)methylphosphonic acid::5'-O-[(S)-HYDROXY(PHOSPHONOMETHYL)PHOSPHORYL]URIDINE::CHEMBL507060::Uridine-5'-alpha,beta-methylene-diphosphate::{[(2R,3S,4R,5R)-5-(2,4-Dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-3,4-dihydroxy-tetrahydro-furan-2-ylmethoxy]-hydroxy-phosphorylmethyl}-phosphonic acid
SMILES: O[C@@H]1[C@@H](COP(O)(=O)CP(O)(O)=O)O[C@H]([C@@H]1O)n1ccc(=O)[nH]c1=O
InChI Key: InChIKey=PDEGDTTUBZXACY-ZOQUXTDFSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ecto-5'-nucleotidase (e5'NT) (Rattus norvegicus (Rat)) | BDBM50271102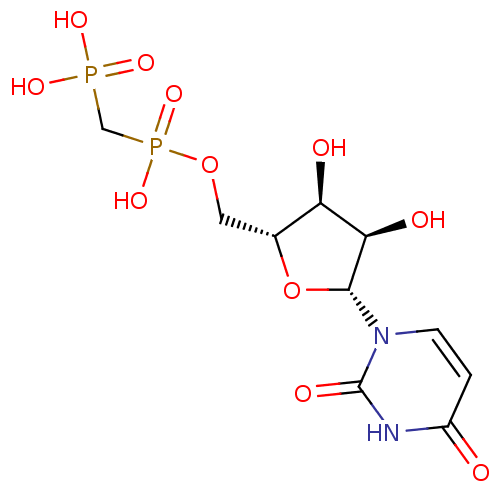 (((((2R,3S,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purinergic receptor P2Y14 (Homo sapiens (Human)) | BDBM50271102 (((((2R,3S,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y14 receptor expressed in HEK293 cells assessed as [3H]inositol phosphate production by scintillation proximi... | J Med Chem 53: 4488-501 (2010) Article DOI: 10.1021/jm100287t BindingDB Entry DOI: 10.7270/Q2RB74SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyrimidinergic receptor P2Y6 (Homo sapiens (Human)) | BDBM50271102 (((((2R,3S,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 339 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in human 1321N1 cells coexpressing phospholipase C-activating Gq protein assessed as [3H]inositol p... | J Med Chem 53: 471-80 (2010) Article DOI: 10.1021/jm901432g BindingDB Entry DOI: 10.7270/Q2W95B4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purinergic receptor P2Y14 (Homo sapiens (Human)) | BDBM50271102 (((((2R,3S,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human P2Y14 receptor expressed in HEK293 cells coexpressing phospholipase C-activating Gi protein cells assessed as inhibition of... | J Med Chem 53: 471-80 (2010) Article DOI: 10.1021/jm901432g BindingDB Entry DOI: 10.7270/Q2W95B4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyrimidinergic receptor P2Y6 (Homo sapiens (Human)) | BDBM50271102 (((((2R,3S,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in human 1321N1 cells assessed as [3H]inositol phosphate production by scintillation pr... | J Med Chem 53: 4488-501 (2010) Article DOI: 10.1021/jm100287t BindingDB Entry DOI: 10.7270/Q2RB74SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyrimidinergic receptor P2Y6 (Homo sapiens (Human)) | BDBM50271102 (((((2R,3S,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 660 | n/a | n/a | n/a | n/a |
NIDDK Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in human 1321N1 cells assessed as [3H]inositol phosphate accumulation by scintillation proximity as... | Bioorg Med Chem 16: 6319-32 (2008) Article DOI: 10.1016/j.bmc.2008.05.013 BindingDB Entry DOI: 10.7270/Q2K07569 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||