

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50274577 2-Methyl-3-(4-[(1-propyl-4-piperidinyl)oxy]phenyl)-4(3H)-quinazolinone::CHEMBL484199
SMILES: CCCN1CCC(CC1)Oc1ccc(cc1)-n1c(C)nc2ccccc2c1=O
InChI Key: InChIKey=JSJYNWJYBPRVNY-UHFFFAOYSA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274577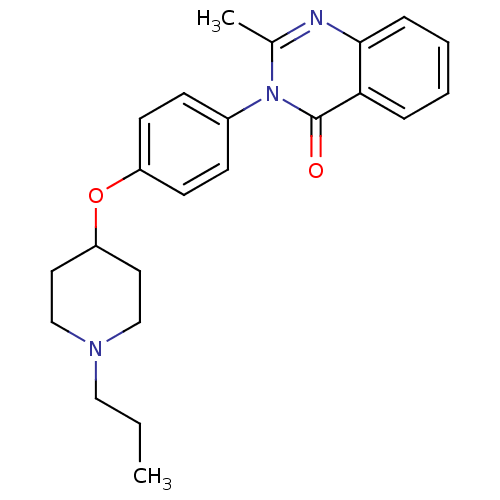 (2-Methyl-3-(4-[(1-propyl-4-piperidinyl)oxy]phenyl)...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||