

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50278411 CHEMBL4170367
SMILES: Cl.[H][C@]12Cc3ccccc3[C@@]1([H])[C@@]2([H])N[C@H]1CC[C@H](N)CC1
InChI Key: InChIKey=XLRHPTGCSRKMLO-DJDKPPBHSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50278411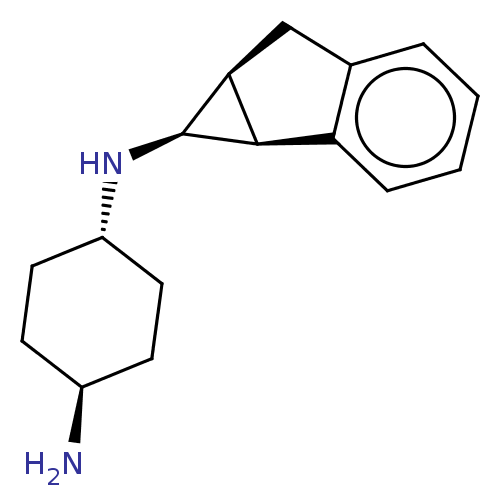 (CHEMBL4170367) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of LSD1 in human MV-4-11 cells assessed as increase in CD86 mRNA expression after 10 days by SYBR Green dye-bsed RT-qPCR analysis | Eur J Med Chem 141: 101-112 (2017) Article DOI: 10.1016/j.ejmech.2017.09.073 BindingDB Entry DOI: 10.7270/Q2474DCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50278411 (CHEMBL4170367) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of binding to human alpha V beta3 | Eur J Med Chem 141: 101-112 (2017) Article DOI: 10.1016/j.ejmech.2017.09.073 BindingDB Entry DOI: 10.7270/Q2474DCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50278411 (CHEMBL4170367) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >20 | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of LSD1 in human MV-4-11 cells assessed as increase in CD86 mRNA expression after 3 days by SYBR Green dye-bsed RT-qPCR analysis | Eur J Med Chem 141: 101-112 (2017) Article DOI: 10.1016/j.ejmech.2017.09.073 BindingDB Entry DOI: 10.7270/Q2474DCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1B (Homo sapiens (Human)) | BDBM50278411 (CHEMBL4170367) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Antagonism of Muscarinic M2 receptors as displacement of concentration response curve of carbachol on isolated guinea-pig left atria | Eur J Med Chem 141: 101-112 (2017) Article DOI: 10.1016/j.ejmech.2017.09.073 BindingDB Entry DOI: 10.7270/Q2474DCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50278411 (CHEMBL4170367) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A expressed in baculovirus infected insect cells by luminescence assay | Eur J Med Chem 141: 101-112 (2017) Article DOI: 10.1016/j.ejmech.2017.09.073 BindingDB Entry DOI: 10.7270/Q2474DCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50278411 (CHEMBL4170367) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His/GST-tagged LSD1 (172 to 852 residues) expressed in baculovirus infected insect cells using biotin-labe... | Eur J Med Chem 141: 101-112 (2017) Article DOI: 10.1016/j.ejmech.2017.09.073 BindingDB Entry DOI: 10.7270/Q2474DCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||