 Found 5 hits for monomerid = 50279591
Found 5 hits for monomerid = 50279591 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50279591
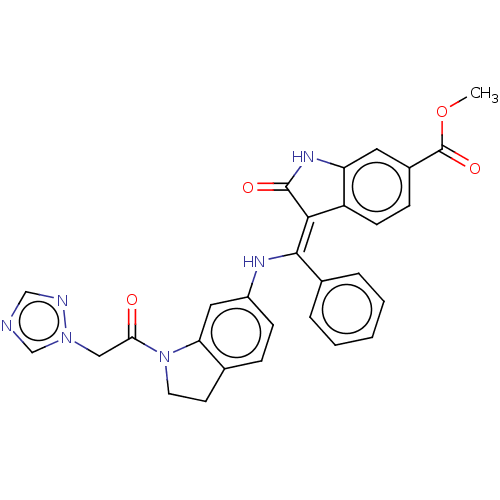
(CHEMBL4172155)Show SMILES COC(=O)c1ccc2\C(=C(\Nc3ccc4CCN(C(=O)Cn5cncn5)c4c3)c3ccccc3)C(=O)Nc2c1 Show InChI InChI=1S/C29H24N6O4/c1-39-29(38)20-8-10-22-23(13-20)33-28(37)26(22)27(19-5-3-2-4-6-19)32-21-9-7-18-11-12-35(24(18)14-21)25(36)15-34-17-30-16-31-34/h2-10,13-14,16-17,32H,11-12,15H2,1H3,(H,33,37)/b27-26- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of dihydrofolate reductase in Lactobacillus casei |
ACS Med Chem Lett 8: 1142-1147 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00164
BindingDB Entry DOI: 10.7270/Q2TM7DND |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Mus musculus (mouse)) | BDBM50279591

(CHEMBL4172155)Show SMILES COC(=O)c1ccc2\C(=C(\Nc3ccc4CCN(C(=O)Cn5cncn5)c4c3)c3ccccc3)C(=O)Nc2c1 Show InChI InChI=1S/C29H24N6O4/c1-39-29(38)20-8-10-22-23(13-20)33-28(37)26(22)27(19-5-3-2-4-6-19)32-21-9-7-18-11-12-35(24(18)14-21)25(36)15-34-17-30-16-31-34/h2-10,13-14,16-17,32H,11-12,15H2,1H3,(H,33,37)/b27-26- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PDGFR in mouse NIH/3T3 cells assessed as reduction in recombinant human PDGF-BB-induced cell proliferation after 42 hrs by cell titer 9... |
ACS Med Chem Lett 8: 1142-1147 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00164
BindingDB Entry DOI: 10.7270/Q2TM7DND |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50279591

(CHEMBL4172155)Show SMILES COC(=O)c1ccc2\C(=C(\Nc3ccc4CCN(C(=O)Cn5cncn5)c4c3)c3ccccc3)C(=O)Nc2c1 Show InChI InChI=1S/C29H24N6O4/c1-39-29(38)20-8-10-22-23(13-20)33-28(37)26(22)27(19-5-3-2-4-6-19)32-21-9-7-18-11-12-35(24(18)14-21)25(36)15-34-17-30-16-31-34/h2-10,13-14,16-17,32H,11-12,15H2,1H3,(H,33,37)/b27-26- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant VEGFR2 (unknown origin) by off-chip mobility shift assay |
ACS Med Chem Lett 8: 1142-1147 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00164
BindingDB Entry DOI: 10.7270/Q2TM7DND |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase component GLS2
(Saccharomyces cerevisiae) | BDBM50279591

(CHEMBL4172155)Show SMILES COC(=O)c1ccc2\C(=C(\Nc3ccc4CCN(C(=O)Cn5cncn5)c4c3)c3ccccc3)C(=O)Nc2c1 Show InChI InChI=1S/C29H24N6O4/c1-39-29(38)20-8-10-22-23(13-20)33-28(37)26(22)27(19-5-3-2-4-6-19)32-21-9-7-18-11-12-35(24(18)14-21)25(36)15-34-17-30-16-31-34/h2-10,13-14,16-17,32H,11-12,15H2,1H3,(H,33,37)/b27-26- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
ACS Med Chem Lett 8: 1142-1147 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00164
BindingDB Entry DOI: 10.7270/Q2TM7DND |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50279591

(CHEMBL4172155)Show SMILES COC(=O)c1ccc2\C(=C(\Nc3ccc4CCN(C(=O)Cn5cncn5)c4c3)c3ccccc3)C(=O)Nc2c1 Show InChI InChI=1S/C29H24N6O4/c1-39-29(38)20-8-10-22-23(13-20)33-28(37)26(22)27(19-5-3-2-4-6-19)32-21-9-7-18-11-12-35(24(18)14-21)25(36)15-34-17-30-16-31-34/h2-10,13-14,16-17,32H,11-12,15H2,1H3,(H,33,37)/b27-26- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FGFR1 (unknown origin) by off-chip mobility shift assay |
ACS Med Chem Lett 8: 1142-1147 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00164
BindingDB Entry DOI: 10.7270/Q2TM7DND |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data