

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50280682 (6R,7S)-2-(2,2-Dimethyl-propionyl)-7-methoxy-3-methyl-5,5-dioxo-5lambda*6*-thia-1-aza-bicyclo[4.2.0]oct-2-en-8-one::CHEMBL274694
SMILES: CO[C@@H]1[C@@H]2N(C1=O)C(C(=O)C(C)(C)C)=C(C)CS2(=O)=O
InChI Key: InChIKey=OJDXKBJOHNWAJF-JOYOIKCWSA-N
Data: 4 Kon
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50280682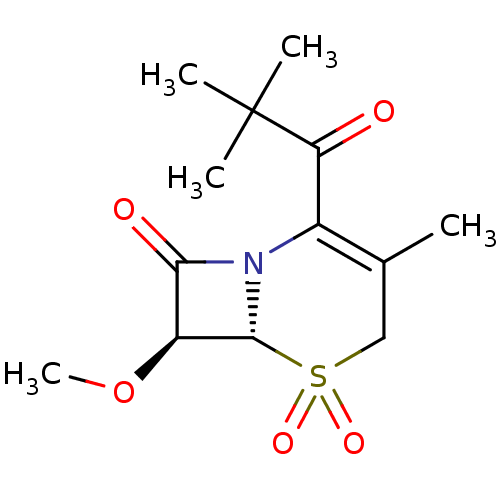 ((6R,7S)-2-(2,2-Dimethyl-propionyl)-7-methoxy-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | n/a | 90 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase II (HLE-II) as second order rate constant | Bioorg Med Chem Lett 2: 1127-1132 (1992) Article DOI: 10.1016/S0960-894X(00)80632-0 BindingDB Entry DOI: 10.7270/Q2HT2P7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50280682 ((6R,7S)-2-(2,2-Dimethyl-propionyl)-7-methoxy-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 90 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50280682 ((6R,7S)-2-(2,2-Dimethyl-propionyl)-7-methoxy-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | n/a | 90 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) expressed as second order inhibition rate constant | Bioorg Med Chem Lett 5: 691-694 (1995) Article DOI: 10.1016/0960-894X(95)00095-B BindingDB Entry DOI: 10.7270/Q2Q2407G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50280682 ((6R,7S)-2-(2,2-Dimethyl-propionyl)-7-methoxy-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | n/a | 90 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) expressed as second order inhibition rate constant | Bioorg Med Chem Lett 5: 687-690 (1995) Article DOI: 10.1016/0960-894X(95)00094-A BindingDB Entry DOI: 10.7270/Q2TT4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||