

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50289258 (S)-2-((S)-3-Carbamoyl-2-{(S)-2-[((R)-2-ethoxycarbonylamino-3-naphthalen-1-yl-propionyl)-methyl-amino]-5-guanidino-pentanoylamino}-propionylamino)-3-methyl-butyric acid methyl ester::CHEMBL80447
SMILES: CCOC(=O)N[C@H](Cc1cccc2ccccc12)C(=O)N(C)[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)OC
InChI Key: InChIKey=WEAHFODXINKIKT-SFIUMABJSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human leukocyte antigen DR beta chain (Homo sapiens (Human)) | BDBM50289258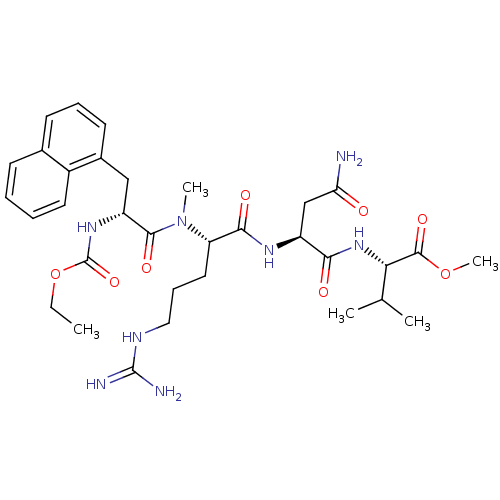 ((S)-2-((S)-3-Carbamoyl-2-{(S)-2-[((R)-2-ethoxycarb...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration required to inhibit the binding of biotinylated rat myelin basic protein peptide (RMBP90-102) against DR1 allele of class II... | Bioorg Med Chem Lett 7: 19-24 (1997) Article DOI: 10.1016/S0960-894X(96)00579-3 BindingDB Entry DOI: 10.7270/Q2Q52PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HLA class II histocompatibility antigen DRB1-1 (Homo sapiens (Human)) | BDBM50289258 ((S)-2-((S)-3-Carbamoyl-2-{(S)-2-[((R)-2-ethoxycarb...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration to inhibit the binding of biotinylated rat myelin basic protein peptide (RMBP90-102) against DR1 allele of class II MHC for ... | Bioorg Med Chem Lett 7: 19-24 (1997) Article DOI: 10.1016/S0960-894X(96)00579-3 BindingDB Entry DOI: 10.7270/Q2Q52PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||