

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50292593 3-oxolanosta-8,24-dien-21-oic acid 21-O-beta-D-xylopyranoside::CHEMBL510510
SMILES: [#6]\[#6](-[#6])=[#6]\[#6]-[#6]-[#6@H](-[#6@H]1-[#6]-[#6][C@@]2([#6])[#6]-3=[#6](-[#6]-[#6][C@]12[#6])[C@@]1([#6])[#6]-[#6]-[#6](=O)C([#6])([#6])[#6@@H]1-[#6]-[#6]-3)-[#6](=O)-[#8]-[#6@@H]-1-[#8]-[#6]-[#6@@H](-[#8])-[#6@H](-[#8])-[#6@H]-1-[#8]
InChI Key: InChIKey=FTPANSKMWIDRLL-QDVPQEPLSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase (cyclooxygenase) (Ovis aries (Sheep)) | BDBM50292593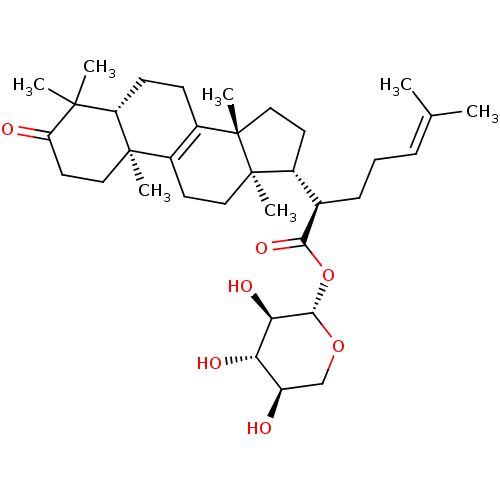 (3-oxolanosta-8,24-dien-21-oic acid 21-O-beta-D-xyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.11E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokushima Bunri University Curated by ChEMBL | Assay Description Inhibition of sheep placental COX2 by liquid scintillation counter | J Nat Prod 68: 69-73 (2005) Article DOI: 10.1021/np040130b BindingDB Entry DOI: 10.7270/Q2S1828J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase (cyclooxygenase) (Ovis aries (Sheep)) | BDBM50292593 (3-oxolanosta-8,24-dien-21-oic acid 21-O-beta-D-xyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.91E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokushima Bunri University Curated by ChEMBL | Assay Description Inhibition of COX1 from ram seminal vesicles by liquid scintillation counter | J Nat Prod 68: 69-73 (2005) Article DOI: 10.1021/np040130b BindingDB Entry DOI: 10.7270/Q2S1828J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||