

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50294237 (4R,7S,10S,13S,16S,19S,22R,25S,28S,31S,34R)-34-amino-31-(4-aminobutyl)-28-benzyl-25-(2-carbamoylethyl)-10-heptyl-13-[(4-hydroxy-3-iodophenyl)methyl]-16-[(1R)-1-hydroxyethyl]-7-(hydroxymethyl)-22-(1H-indol-3-ylmethyl)-6,9,12,15,18,21,24,27,30,33-decaoxo-19-({4-[(propan-2-ylamino)methyl]phenyl}methyl)-1,2-dithia-5,8,11,14,17,20,23,26,29,32-decaazacyclopentatriacontane-4-carboxamide::CHEMBL540721
SMILES: CCCCCCC[C@@H]1NC(=O)[C@H](Cc2ccc(O)c(I)c2)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccc(CNC(C)C)cc2)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(N)=O)[C@@H](C)O
InChI Key: InChIKey=MQCRXSDRFFCGNP-SNBDFQEWSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50294237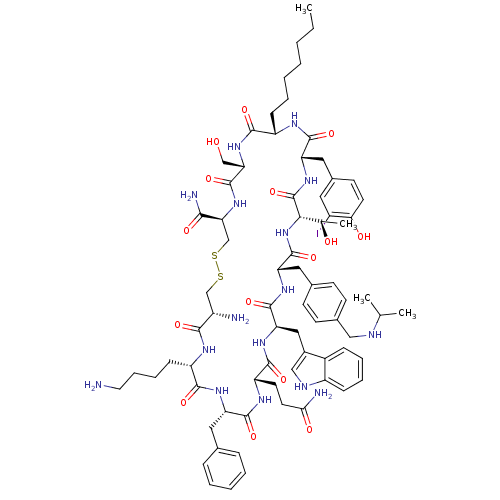 ((4R,7S,10S,13S,16S,19S,22R,25S,28S,31S,34R)-34-ami...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Clayton Foundation Laboratories for Peptide Biology Curated by ChEMBL | Assay Description Displacement of [125I]LTT-SRIF-28 from human sst3 receptor by autoradiography | J Med Chem 52: 2733-46 (2009) Article DOI: 10.1021/jm801314f BindingDB Entry DOI: 10.7270/Q2CV4JPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50294237 ((4R,7S,10S,13S,16S,19S,22R,25S,28S,31S,34R)-34-ami...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Clayton Foundation Laboratories for Peptide Biology Curated by ChEMBL | Assay Description Displacement of [125I]LTT-SRIF-28 from human sst4 receptor by autoradiography | J Med Chem 52: 2733-46 (2009) Article DOI: 10.1021/jm801314f BindingDB Entry DOI: 10.7270/Q2CV4JPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 1 (Homo sapiens (Human)) | BDBM50294237 ((4R,7S,10S,13S,16S,19S,22R,25S,28S,31S,34R)-34-ami...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Clayton Foundation Laboratories for Peptide Biology Curated by ChEMBL | Assay Description Displacement of [125I]LTT-SRIF-28 from human sst1 receptor by autoradiography | J Med Chem 52: 2733-46 (2009) Article DOI: 10.1021/jm801314f BindingDB Entry DOI: 10.7270/Q2CV4JPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50294237 ((4R,7S,10S,13S,16S,19S,22R,25S,28S,31S,34R)-34-ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Clayton Foundation Laboratories for Peptide Biology Curated by ChEMBL | Assay Description Displacement of [125I]LTT-SRIF-28 from human sst2 receptor by autoradiography | J Med Chem 52: 2733-46 (2009) Article DOI: 10.1021/jm801314f BindingDB Entry DOI: 10.7270/Q2CV4JPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50294237 ((4R,7S,10S,13S,16S,19S,22R,25S,28S,31S,34R)-34-ami...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Clayton Foundation Laboratories for Peptide Biology Curated by ChEMBL | Assay Description Displacement of [125I]LTT-SRIF-28 from human sst5 receptor by autoradiography | J Med Chem 52: 2733-46 (2009) Article DOI: 10.1021/jm801314f BindingDB Entry DOI: 10.7270/Q2CV4JPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||