

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CN1[C@@H]2CC[C@H]3[C@@H]4C\C(=C/c5cncnc5)[C@H](O)[C@@]4(C)CC[C@@H]3[C@@]2(C)C=CC1=O
InChI Key: InChIKey=GJFAJAHCWGSYHL-GYXROJBQSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296943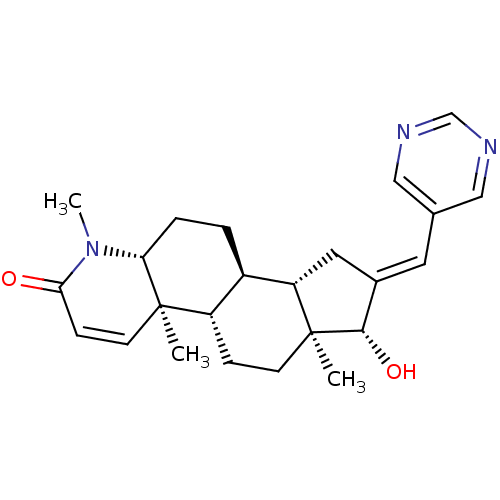 (16-(Pyrimidin-5-ylmethylidene)-17beta-hydroxy-4-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG potassium channel | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296943 (16-(Pyrimidin-5-ylmethylidene)-17beta-hydroxy-4-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in human MDA-MB-453 cells transfected with MMTV-LUC assessed as induction of MMTV-LTR/promoter linked LUC gene ... | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50296943 (16-(Pyrimidin-5-ylmethylidene)-17beta-hydroxy-4-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of estrogen receptor | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50296943 (16-(Pyrimidin-5-ylmethylidene)-17beta-hydroxy-4-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mineralocorticoid receptor | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50296943 (16-(Pyrimidin-5-ylmethylidene)-17beta-hydroxy-4-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of progesterone receptor | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296943 (16-(Pyrimidin-5-ylmethylidene)-17beta-hydroxy-4-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50296943 (16-(Pyrimidin-5-ylmethylidene)-17beta-hydroxy-4-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of glucocorticoid receptor | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||