

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50300549 2,3-Dihydroxy-9,10-dimethoxy-5,6-dihydro-isoquino[3,2-a]isoquinolinylium chloride::CHEMBL575979
SMILES: COc1ccc2cc3-c4cc(O)c(O)cc4CC[n+]3cc2c1OC
InChI Key: InChIKey=HVTCKKMWZDDWOY-UHFFFAOYSA-O
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell division control protein 42 homolog (Homo sapiens (Human)) | BDBM50300549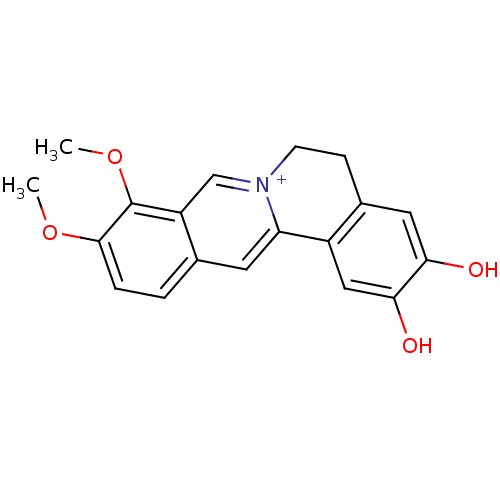 (2,3-Dihydroxy-9,10-dimethoxy-5,6-dihydro-isoquino[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonhit Therapeutics Curated by ChEMBL | Assay Description Inhibition of Cdc42 GTPase activity assessed as incorporation of BODIPY-GTP after 40 mins by nucleotide binding competition assay | Bioorg Med Chem Lett 19: 5594-8 (2009) Article DOI: 10.1016/j.bmcl.2009.08.037 BindingDB Entry DOI: 10.7270/Q27P8ZF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50300549 (2,3-Dihydroxy-9,10-dimethoxy-5,6-dihydro-isoquino[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of human CYP1B1 expressed in Escherichia coli DH5alpha coexpressing human NADPH-P450 reductase using 4-estradiol as substrate in presence ... | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ras-related C3 botulinum toxin substrate 1/T-lymphoma invasion and metastasis-inducing protein 1 (Homo sapiens (Human)) | BDBM50300549 (2,3-Dihydroxy-9,10-dimethoxy-5,6-dihydro-isoquino[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonhit Therapeutics Curated by ChEMBL | Assay Description Inhibition of Rac1 GTPase activity assessed as incorporation of BODIPY-GTP after 40 mins by nucleotide binding competition assay | Bioorg Med Chem Lett 19: 5594-8 (2009) Article DOI: 10.1016/j.bmcl.2009.08.037 BindingDB Entry DOI: 10.7270/Q27P8ZF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||