

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50303323 (2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)carbonyl]-amino}ethyl)phosphinyl]-(2R)-2-[(3-phenylisoxazol-5-yl)methyl]-1-oxopropyl}amino)1H-Indole-3-propanoic Acid::CHEMBL577754
SMILES: OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1
InChI Key: InChIKey=ITMFWUAKJPURIP-NINLVYDZSA-N
Data: 8 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50303323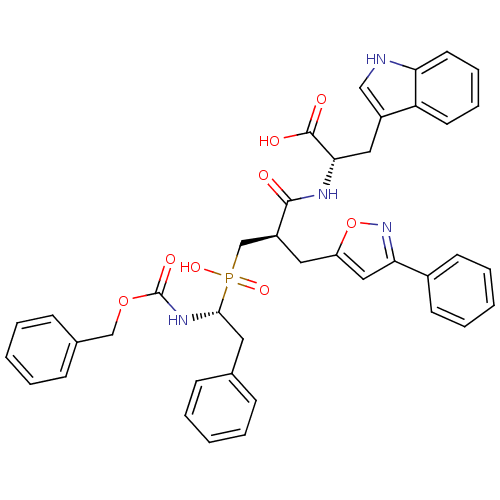 ((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic ACE C-terminal domain | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (ECE1) (Homo sapiens (Human)) | BDBM50303323 ((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic ECE1 | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50303323 ((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic ACE in presence of buffer | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50303323 ((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic ACE in presence of 1:100 diluted SHR rat plasma | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50303323 ((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human MMP13 | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50303323 ((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic ACE in presence of 1:100 diluted SHR rat plasma complemented with 5 uM serum albumin | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50303323 ((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic ACE in presence of 1:50 diluted SHR rat plasma | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50303323 ((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic NEP | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||