

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50304144 5-NITROINDAZOLE::5-Nitro-1H-indazole::CHEMBL165372
SMILES: [O-][N+](=O)c1ccc2[nH]ncc2c1
InChI Key: InChIKey=WSGURAYTCUVDQL-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50304144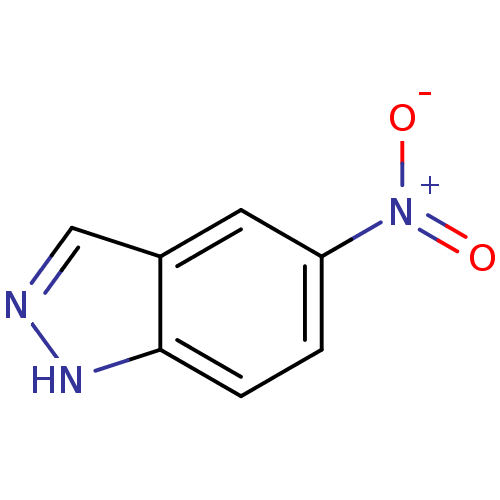 (5-NITROINDAZOLE | 5-Nitro-1H-indazole | CHEMBL1653...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 5.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged PDE10A2 catalytic domain expressed in Escherichia coli BL21(DE3) RIL cells using 3',5'-cGMP as substrate b... | J Med Chem 59: 7029-65 (2016) Article DOI: 10.1021/acs.jmedchem.5b01813 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50304144 (5-NITROINDAZOLE | 5-Nitro-1H-indazole | CHEMBL1653...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 5.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged PDE10A2 catalytic domain expressed in Escherichia coli BL21(DE3) RIL cells using 3',5'-cGMP as substrate b... | J Med Chem 59: 7029-65 (2016) Article DOI: 10.1021/acs.jmedchem.5b01813 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50304144 (5-NITROINDAZOLE | 5-Nitro-1H-indazole | CHEMBL1653...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus-infected insect cells using p-tyraimne substrate | J Med Chem 57: 6679-703 (2014) Article DOI: 10.1021/jm500729a BindingDB Entry DOI: 10.7270/Q2HT2R0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50304144 (5-NITROINDAZOLE | 5-Nitro-1H-indazole | CHEMBL1653...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED Curated by ChEMBL | Assay Description Inhibition of NOS1 | Bioorg Med Chem 17: 6180-7 (2009) Article DOI: 10.1016/j.bmc.2009.07.067 BindingDB Entry DOI: 10.7270/Q2ZG6SBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||