

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50304619 (S)-2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)-2-hydroxypropanoyl)piperidin-4-yl)-5-methyl-1H-imidazo[1,5-c]imidazol-3(2H)-one::2-(1-{(2S)-3-[(6-Chloronaphthalene-2-yl)sulfonyl]-2-hydroxypropanoyl}piperidin-4-yl)-5-methyl-1,2-dihydro-3Himidazo[1,5-c]imidazol-3-one::CHEMBL593461
SMILES: Cc1ncc2CN(C3CCN(CC3)C(=O)[C@H](O)CS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C(=O)n12
InChI Key: InChIKey=WSDPDXBHHQAFEA-JOCHJYFZSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM50304619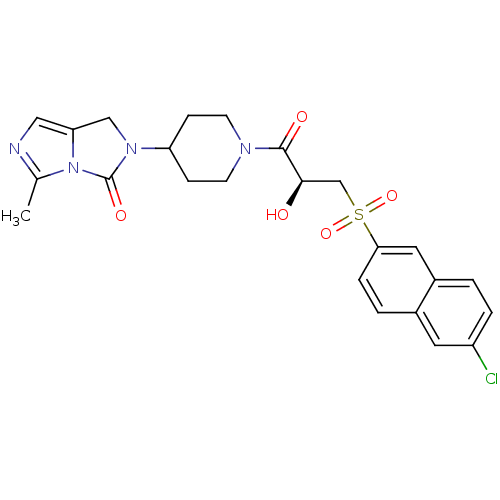 ((S)-2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)-2-hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of factor 10a | J Med Chem 53: 6243-74 (2010) Article DOI: 10.1021/jm100146h BindingDB Entry DOI: 10.7270/Q2CR5VBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50304619 ((S)-2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)-2-hy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of human thrombin by para-nitroanilide release assay | Bioorg Med Chem 17: 7993-8002 (2009) Article DOI: 10.1016/j.bmc.2009.10.009 BindingDB Entry DOI: 10.7270/Q2HX1DM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50304619 ((S)-2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)-2-hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of human plasmin by para-nitroanilide release assay | Bioorg Med Chem 17: 7993-8002 (2009) Article DOI: 10.1016/j.bmc.2009.10.009 BindingDB Entry DOI: 10.7270/Q2HX1DM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50304619 ((S)-2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)-2-hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of human tissue plasminogen activator by para-nitroanilide release assay | Bioorg Med Chem 17: 7993-8002 (2009) Article DOI: 10.1016/j.bmc.2009.10.009 BindingDB Entry DOI: 10.7270/Q2HX1DM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50304619 ((S)-2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)-2-hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of human factor 10a assessed as p-nitroanilide release using S2765 as substrate by chromogenic assay | J Med Chem 53: 3517-31 (2010) Article DOI: 10.1021/jm901699j BindingDB Entry DOI: 10.7270/Q2PR7W52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50304619 ((S)-2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)-2-hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of human factor 10a by para-nitroanilide release assay | Bioorg Med Chem 17: 7993-8002 (2009) Article DOI: 10.1016/j.bmc.2009.10.009 BindingDB Entry DOI: 10.7270/Q2HX1DM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||