

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50304793 (S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-1-oxo-3-phenylpropan-2-ylcarbamate::CHEMBL604281
SMILES: CN(C#N)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1
InChI Key: InChIKey=FTJHEEIVOBBMMC-SFHVURJKSA-N
Data: 6 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Homo sapiens (Human)) | BDBM50304793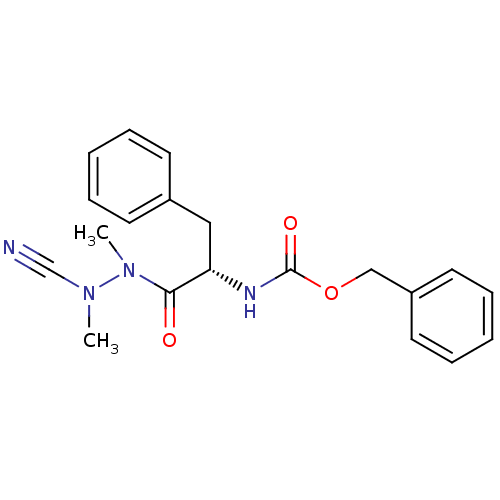 ((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-1-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50304793 ((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-1-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50304793 ((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-1-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin S after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50304793 ((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-1-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin B after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50304793 ((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-1-ox...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50304793 ((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-1-ox...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma cruzi cruzain using Z-phe-Arg-7-amido-4-methylcoumarin as substrate by fluorometric method | Bioorg Med Chem Lett 27: 5031-5035 (2017) Article DOI: 10.1016/j.bmcl.2017.10.002 BindingDB Entry DOI: 10.7270/Q2125W7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50304793 ((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-1-ox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description Inhibition of Carica papaya papain by microtiter plate spectrofluorimetry | Bioorg Med Chem Lett 20: 252-5 (2010) Article DOI: 10.1016/j.bmcl.2009.10.122 BindingDB Entry DOI: 10.7270/Q24X57WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM50304793 ((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-1-ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||