

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Oc1cccc(Nc2ncc3CC(=O)Nc4cccnc4-c3n2)c1
InChI Key: InChIKey=DKMQQYBCJVFNRW-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50308205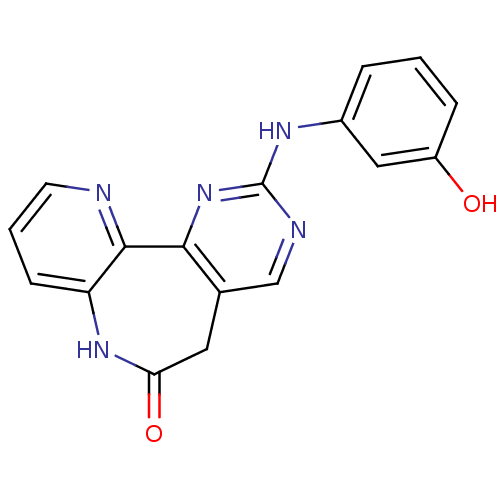 (2-(3-Hydroxyanilino)-5,7-dihydro-6H-pyrido[3,2-b]p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universitat Braunschweig Curated by ChEMBL | Assay Description Inhibition of PLK1 assessed as [33Pi] incorporation by microplate scintillation counting in presence of 1 uM ATP | J Med Chem 53: 2433-42 (2010) Article DOI: 10.1021/jm901388c BindingDB Entry DOI: 10.7270/Q24J0G22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50308205 (2-(3-Hydroxyanilino)-5,7-dihydro-6H-pyrido[3,2-b]p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universitat Braunschweig Curated by ChEMBL | Assay Description Inhibition of VEGFR2 assessed as [33Pi] incorporation by microplate scintillation counting in presence of 1 uM ATP | J Med Chem 53: 2433-42 (2010) Article DOI: 10.1021/jm901388c BindingDB Entry DOI: 10.7270/Q24J0G22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50308205 (2-(3-Hydroxyanilino)-5,7-dihydro-6H-pyrido[3,2-b]p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universitat Braunschweig Curated by ChEMBL | Assay Description Inhibition of INSR assessed as [33Pi] incorporation by microplate scintillation counting in presence of 1 uM ATP | J Med Chem 53: 2433-42 (2010) Article DOI: 10.1021/jm901388c BindingDB Entry DOI: 10.7270/Q24J0G22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||