

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCOC[C@@H]1[C@H]2CNC[C@@]12c1ccc(Cl)c(Cl)c1
InChI Key: InChIKey=IBZJKEOJOGAMGJ-GYSYKLTISA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (Rattus norvegicus (rat)) | BDBM50308250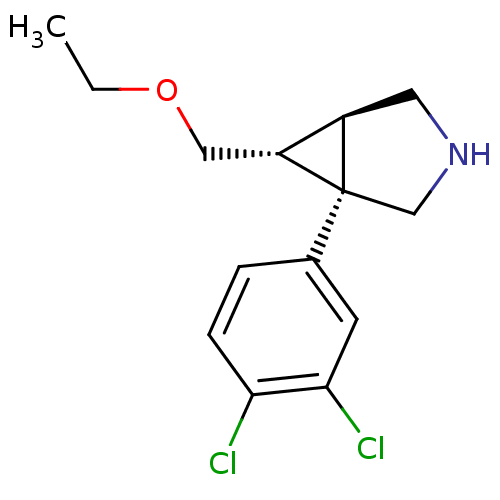 ((1R,5R,6R)-1-(3,4-dichlorophenyl)-6-(ethoxymethyl)...) | Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]nisoxetine from rat hippocampus NET by filtration binding assay | J Med Chem 53: 2534-51 (2010) Article DOI: 10.1021/jm901818u BindingDB Entry DOI: 10.7270/Q2ST7PX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (MOUSE) | BDBM50308250 ((1R,5R,6R)-1-(3,4-dichlorophenyl)-6-(ethoxymethyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from mouse cortex SERT by filtration binding assay | J Med Chem 53: 2534-51 (2010) Article DOI: 10.1021/jm901818u BindingDB Entry DOI: 10.7270/Q2ST7PX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50308250 ((1R,5R,6R)-1-(3,4-dichlorophenyl)-6-(ethoxymethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre Curated by ChEMBL | Assay Description Inhibition of histamine H1 receptor | J Med Chem 53: 2534-51 (2010) Article DOI: 10.1021/jm901818u BindingDB Entry DOI: 10.7270/Q2ST7PX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50308250 ((1R,5R,6R)-1-(3,4-dichlorophenyl)-6-(ethoxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre Curated by ChEMBL | Assay Description from Dfrom ifrom sfrom pfrom lfrom afrom cfrom efrom mfrom efrom nfrom tfrom from ofrom ffrom from [from 3from Hfrom ]from dfrom ofrom ffrom efrom tf... | J Med Chem 53: 2534-51 (2010) Article DOI: 10.1021/jm901818u BindingDB Entry DOI: 10.7270/Q2ST7PX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||